INTRODUCTION
Urinary tract obstruction is a serious and common clinical condition associated with increased intraluminal pressure in the ureter and renal tubules that can cause renal parenchymal damage through a series of direct and indirect effects [1]. Although the obstruction is potentially reversible with treatment, marked and sometimes prolonged diuresis associated with an impaired ability to concentrate urine may follow relief of the obstruction [2].
The recent discovery of aquaporin (AQP) channels has increased our understanding of water transport across the permeable epithelial cell membrane. AQPs are a family of transmembrane proteins that selectively allow water and/or other small, uncharged molecules, such as urea and glycerol (aquaglyceroporins), to pass along hydrostatic and osmotic gradients [3-5]. A total of 13 different mammalian AQPs have been identified at the molecular level and localized to particular tissues. Of these isoforms, Aquaporin-3 (AQP3, originally known as glycerol intrinsic protein) has a glycerol-transport function and is primarily responsible for the water channel in the basolateral plasma membranes of epithelial cells in the collecting ducts of the kidney [6].
Complete ureteric obstruction has been widely investigated, and an abundance of pathophysiological data are available. In addition, it is relatively well known that the expression of AQP is decreased when there is complete ureteral obstruction. In clinical practice, however, the problem of partial obstruction is considerably more common. Furthermore, unilateral ureteral obstruction is characterized by several renophysiological, morphologic, and volumetric changes. It has been reported that not only the obstructed kidney, but also the complementary kidney, undergoes functional changes in rats with unilateral ureteral obstruction [7]. Despite this evidence, there has been a paucity of studies on the molecular mechanisms associated with UPUO. Therefore, our aim was to investigate AQP3 expression in UPUO and the intracellular changes in the kidney of rats with UPUO. This would increase our understanding of the role of AQP3, especially in the clinical setting of UPUO.
MATERIALS AND METHODS
1. Animals
The animals used in this study were Sprague-Dawley rats (200-250 g; Daehan Biolink Co., Daejeon, Korea) that were raised in pairs in cages covered with sawdust. Water and food were available ad libitum, the temperature was controlled at 22℃, and the illumination was changed at 12-hour intervals. A total of 30 rats were divided into two groups: the UPUO group (n=20) and the control group (n=10).
2. Experimental surgery for UPUO
In the control group, the rats were anesthetized through an injection of 15 mg ketamine/kg (Yuhan Co., Seoul, Korea) and 5 mg xylazine/kg (Rompun, Bayer Korea Co., Seoul, Korea) intraperitoneally, which was followed by a sham operation. In the UPUO group, partial obstruction of the left ureter was induced by using a modified version of the method used by Modi et al. [8]. Details of the experimental surgery were as follows: after the left ureter was exposed through a median incision of the abdomen under anesthesia, a 4-mm long silicone tube was inserted through an incision on the ureter of about half the length of the tube. Cases without gross hydronephrosis or for which concomitant complete ureteral obstruction was found were excluded from the study. To check for partial ureteral obstruction, indigocarmine was injected in the renal pelvis, which presented in the ureter inferior to the surgical site. Nephrectomies of the left kidneys were performed in both groups 7 days after the operation.
3. Histologic examination and immunofluorescent staining
For histologic examination, the kidneys were fixed with 10% neutral formalin, washed with flowing water to remove fixative, and dehydrated. Kidney tissue samples were then embedded in paraffin, sectioned into 4-µm thick slices, and bonded to glass slides coated with saline. After clearing (paraffin removal), the slides were stained with hematoxylin and eosin. The thickness of the epithelial cells, dilation of the collecting ducts/distal tubules, and infiltration of inflammatory cells were compared between samples.
The deparaffinization and hydration procedure was as follows: the slides were reacted with Xylene I and II for 10 minutes; exposed to 100%, 95%, 90%, 80%, and 70% alcohol (in that order) for 5 minutes each; washed with distilled water for 5 minutes; and washed with 0.01 M (pH 7.4) phosphate-buffered saline (PBS) three times for 10 minutes. To expose the antigens, the slides were dipped into 1 X sodium citrate buffer solution before being heated in a microwave oven for 4 minutes and then washed with 0.01 M (pH 7.4) PBS three times for 10 minutes. To prevent non-specific reactions, the slides were treated with 10% normal goat serum at 37℃ for 1 hour. They were then exposed to AQP3 (H-80) rabbit polyclonal antibody (1:400; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) as the primary antibody at 4℃, left overnight, and then washed with 0.01 M (pH 7.4) PBS three times for 10 minutes. Goat antirabbit immunoglobulin G fluorescein isothiocyanate (1:400; Santa Cruz Biotechnology Inc.) and 4',6-diamidino-2-phenyindole dilactate (1:400; Sigma Aldrich Co., St. Louis, MO, USA) were used as secondary antibodies and for staining of the nucleus. The slides were washed with 0.01 M PBT (PBS+0.1% Triton X-100; Sigma-Aldrich Co.,) twice for 30 minutes and then washed with PBS three times for 20 minutes. After being stained, the slides were mounted by using mounting media (Dako, Carpinteria, CA, USA). The expression of AQP3 fluorescence was estimated by using a confocal microscope (LSM 710, Carl Zeiss, Jena, Germany) and image analyzer (Zeiss LSM 5.0, Carl Zeiss, Jena, Germany).
RESULTS
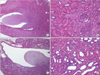
1. Comparison of histologic findings between the experimental and control groups
Examination of the samples stained with hematoxylin and eosin showed dilation of the renal pelvis and ureter above the obstructed site, thinning of the epithelial layer, dilation of the collecting ducts/distal tubules, and infiltration of inflammatory cells in the UPUO group but not in the control group (Fig. 1).
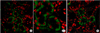
2. Localization and detection of changes in AQP3 expression by immunofluorescent staining
AQP3 was mainly located in the basolateral aspect of principal cells (A-1, magnified A, ×680). The immunoreactive expression of AQP3 was significantly weaker in the UPUO group than in the control group (Fig. 2), and the mean optical density of AQP3 was significantly lower in the UPUO group than in the control group (100.90±17.52 compared with 131.67±16.86, p<0.001) (Fig. 3).
DISCUSSION
AQPs are small plasma integral membrane proteins that play a role in several cellular functions, such as osmolarity control and water movement across the urothelium. The AQP subtype AQP3 plays an important role in facilitating water and glycerol transport across the membrane of the urothelium. AQP3 cDNA in rats has been isolated and encodes a 292-amino acid water/glycerol-transporting glycol protein expressed in the kidney, large airway, eye, urinary bladder, skin, and gastrointestinal tract [9]. However, the AQP3 transcript and protein are most strongly expressed in the kidney. AQP3 is abundant in the connecting tubule and in the cortical, outer medullary, and inner medullary collecting duct [10,11]. At the subcellular level, AQP3 is abundant in both the basal and lateral plasma membranes of the principal cells in the collecting duct [12].
A study of all 13 AQP subtypes in normal human urothelial cells and in human tissues of the bladder and ureters reported the expression of AQP3, AQP4, AQP7, AQP9, and AQP11 but not of AQP0, AQP1, AQP2, AQP5, AQP6, AQP8, AQP10, or AQP12 [5]. In human urothelium, AQP3 was strongly expressed at the cell borders of the basal and intermediate layers in both urothelium in situ and in differentiated tissue constructed in vitro [5]. It is suggested that AQP3 may play a role in the transport of transurothelial water and solutes.
Recently, it was reported that AQP3 plays a vital role in the countercurrent multiplier mechanism by allowing efficient osmotic water equilibration [13-15]. AQP2 is known to play a role in both the short- and long-term-regulation of the AVP/cAMP pathway to increase the water permeability of the apical membrane. A regulatory role in the AVP/cAMP pathway on AQP3 channels has also been shown [16]. Therefore, decreased cAMP formation may result in a decrease in AQP channels regulated by the AVP/cAMP pathway. It is well known that the deletion of AQP3 in the kidney produces marked polyuria and that AQP3 knockout mice are able to generate only partially concentrated urine after water deprivation [9]. Similarly to the findings in Wistar rats, AQP3 abundance was also reduced in Brattleboro rats, which indicates that vasopressin-independent mechanisms are involved in AQP3 regulation.
The pathophysiology of renal failure in complete ureteric obstruction has been shown to have a dual mechanism. The initial increase in renal tract pressure through the renal pelvis is transient and returns to normal within 12 hours [17]. If obstruction continues, a phase of increased renal blood flow is followed by secondary hypoperfusion. This reduction in blood flow persists if obstruction continues for more than 24 to 36 hours and thus limits the recovery of renal function. Therefore, it appears that surgical decompression of the upper tract must be performed before the onset of the secondary vascular injury if permanent damage is to be avoided. The development of impaired renal function in partial ureter obstruction follows the same mechanism, as would be expected, but at a slower rate.
Li et al. [18] studied bilateral ureteral obstruction (BUO) and found that the release of BUO is associated with the down-regulation of AQP3. On the basis of their results, the reduced levels of AQP3 observed up to 14 days after release of BUO suggest that a reduction in AQP3 may also play a significant role in urinary concentrating defects after the release of BUO.
Wang et al. [19] reported on the results of long-term follow-up in rats with neonatally induced UPUO. AQP3 expression was increased in partially obstructed kidneys 7 weeks after induction, which may have been a compensatory phenomenon, but was decreased 14 weeks after induction. These findings suggest a time- and age-dependent dysregulation in response to UPUO. AQP3 expression may be influenced by age and the duration and degree of obstruction. In the current study, we observed thinning of the epithelial layer, dilation of the collecting ducts/distal tubules, infiltration of inflammatory cells, and significantly decreased AQP3 expression in the collecting ducts/distal tubules in the UPUO group. These findings suggest that undefined mechanisms may be responsible for the decreased phosphorylation and increased abundance of AQP3. These mechanisms should be investigated further in future studies. The results of our study suggest that AQP3 is influenced by cell damage induced by local/intrarenal factors in the early phase of partial obstruction. The observed down-regulation of water channels in the collecting duct can be attributed to AQP3, which may provide a molecular explanation for the functional defects previously shown in the collecting duct. The reduction in AQP3 in rats with UPUO may contribute, at least partially, to impaired water metabolism and to a reduction in the net reabsorption of water at several nephronic sites in the obstructed kidney, including the proximal straight tubule, descending thin limb, inner stripe of the outer medullary collecting duct, and inner medullary collecting duct.
CONCLUSIONS
The results are consistent with a urinary concentration defect in the obstructed kidney, which suggests a functional association between histologic changes and the ability of the obstructed kidney to handle sodium and water in a UPUO model. These findings suggest that AQP3 may play a pathophysiological role in UPUO. Therefore, it is important that we promptly treat acute renal tissue damage induced by conditions commonly seen in clinical practice, such as ureteral stones and obstructions.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download