Abstract
Purpose
Klinefelter syndrome is a chromosomal disorder present in 1 out of 400 to 1,000 male newborns in Western populations. Two-thirds of affected newborns show a karyotype of 47,XXY. Few studies have examined the incidence of Klinefelter syndrome in Korea. The aim of this study was to investigate the incidence of Klinefelter syndrome by use of prenatal screening tests.
Materials and Methods
From January 2001 to December 2010, 18,049 pregnant women who had undergone a chromosomal study for fetal anomalies were included. For fetuses that were diagnosed as having Klinefelter syndrome, the patients' medical records were retrospectively reviewed. Both parents' ages, the reason for the chromosomal studies, and karyotypes were investigated.
Results
We found that 22 of 18,049 (0.12%) fetuses were diagnosed with Klinefelter syndrome. The incidence of this disorder in male fetuses was 22 of 9,387 (0.23%). Also, 19 of the newborns (86.4%) showed a karyotype of 47,XXY; the other newborns showed karyotypes of 48,XXY,+21; 48,XXY,+12[12]/46,XY[54]; and 47,XXY[6]/45,X[1]/46,XY[95]. The mean age of the mothers was 36.1 years, and 2 women had a past history of a Down syndrome pregnancy. Nine mothers had a normal spontaneous delivery, 9 mothers underwent artificial abortion, and 2 fetuses were spontaneously aborted.
Klinefelter syndrome was first described in 1942 as an endocrine disorder caused by a supernumerary X chromosome that is characterized by gynecomastia, small testes, absent spermatogenesis, normal to moderately reduced Leydig cell function, and increased secretion of follicle-stimulating hormone [1,2]. Klinefelter syndrome is the most common sex-chromosome disorder and affects 1/500 persons [3,4]. The prevalence of Klinefelter syndrome was reported to be 0.1% to 0.2% in the general population and 0.15% to 0.17% in prenatal diagnoses [4-8]. Klinefelter syndrome is the most frequent genetic disorder of male infertility; it is present in 10% of azoospermic men [9].
The 47,XXY karyotype is almost always the result of meiotic nondisjunction during parental gamete formation, which increases with both maternal and paternal age [10,11]. A recent study reported that the prevalence of Klinefelter syndrome is increasing [12]. However, Klinefelter syndrome is diagnosed prenatally and rarely postnatally because chromosomal studies are not routinely performed. Also, at birth, most 47,XXY neonates appear normal.
Patients who are diagnosed with Klinefelter syndrome are often seen at an infertility clinic, but there are no studies on the prevalence of Klinefelter syndrome in the Korean population. We therefore investigated the incidence of Klinefelter syndrome after amniocentesis or chorionic villi biopsy.
This was a retrospective study of 18,043 mothers who visited Cheil General Hospital & Women's Healthcare Center and had amniocentesis, cord blood collection, or chorionic villi biopsy from January 2001 to December 2010. The institutional review board approved the study. Indications for chromosomal studies were as follows: advanced maternal age (≥35 years); previous child with a de novo chromosomal abnormality; presence of a structural chromosomal abnormality in one of the parents; family history of a genetic disorder that could be diagnosed or ruled out by biochemical or DNA analysis; 2 continuous abortions or more than 3 abortions without reason; birth to a stillborn with unknown cause; chromosomal abnormality of the mother, father, or close relatives; history of birth to a congenitally abnormal baby; fetus malformation detected by ultrasonography; positive results on a test for neural tube defects or Down syndrome by use the mother's serum; and a wish for such tests by the mother.
For fetuses that were diagnosed with Klinefelter syndrome, the mothers' and fathers' ages, the reason the chromosomal studies were performed, and karyotypes were investigated. Also, the medical records of fetuses with Klinefelter syndrome were reviewed.
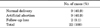
In this study, 22 of 18,049 fetuses (0.12%) were diagnosed with Klinefelter syndrome. The incidence of this disorder in male fetuses was 0.23% (22/9387). The mean age of the mothers and fathers of the affected fetuses was 35.5 years (range, 29 to 47 years) and 38 years (range, 32 to 51 years), respectively. Also, 19 fetuses (86.4%) showed a karyotype of 47,XXY; the other three fetuses had karyotypes of 48,XXY,+21; 48,XXY,+12[12]/46,XY[54]; and 47,XXY[6]/45,X[1]/46,XY[95], respectively (Table 1).
The most common indication for chromosomal study was advanced maternal age (>35 years), and there were 16 mothers (72.7%) in this group. The next most common indicators were history of chromosomal abnormal birth (3 mothers, 13.6%), fetus malformation detected by ultrasonography (2 mothers, 9.1%), and chromosomal abnormality of the parent (1 mother, 4.5%) (Table 2).
In this study, 9 mothers (40.9%) had a normal delivery, 9 mothers (40.9%) chose artificial abortion, and 2 fetuses (9.1%) were spontaneously aborted (Table 3).
Klinefelter syndrome is a common sex-chromosome disorder in which males are born with an extra copy of the X chromosome. It occurs at a frequency of approximately 1 in 500 men and results in impaired spermatogenesis and androgen deficiency [13]. About 80% of cases are due to the congenital numerical chromosome aberration 47,XXY; the remaining 20% have higher-grade chromosome aneuploidies (48,XXXY; 48,XXYY; 49,XXXXY), 46,XY/47,XXY mosaicism, or structurally abnormal X chromosomes [14,15]. Our study also showed that 86.4% of Klinefelter fetuses had the 47,XXY karyotype.
Klinefelter syndrome subjects are traditionally described as infertile, and semen analysis most often reveals azoospermia. Klinefelter syndrome occurs in about 10% of azoospermic men and 4% of infertile men [16]. The only way for pregnancy in Klinefelter syndrome couples is testicular sperm extraction (TESE) combined with intracytoplasmic sperm injection. Also, microsurgical multiple TESE (m-TESE) has shown significantly better sperm recovery rates than conventional TESE [17].
A recent study suggested that the prevalence of Klinefelter syndrome may be increasing [12]. One reason is that the 47,XXY karyotype is almost always the result of meiotic nondisjunction during parental gamete formation, which increases with both maternal and paternal age [10,11]. Interest in infertility has increased recently because the mother's age has increased. In our study, the average age of the mothers who had a fetus diagnosed with Klinefelter syndrome was 36.1 years and the reason that the tests were performed was the mother's advanced age.
Diagnosis of Klinefelter syndrome is often delayed because of substantial variation in clinical presentation. Abramsky and Chapple calculated that 10% of expected cases were identified prenatally and 26% were diagnosed in childhood or adult life because of hypogonadism, gynecomastia, or infertility, leaving 64% of cases undiagnosed [18]. This is because although Klinefelter syndrome can be diagnosed by amniocentesis or chorionic villi biopsy, these tests are not routinely performed. Small, firm testes and variable symptoms of androgen deficiency characterize Klinefelter syndrome males and are most often detected among patients with azoospermia visiting infertility clinics [19,20]. The low diagnosis rate suggests that most males with Klinefelter syndrome will not receive potentially beneficial treatments, especially androgen therapy. Detection in childhood and timely intervention may be necessary for optimal medical and psychosocial outcomes in adulthood [21].
Prior to 2009 when the mother and child health law declared abortions of Klinefelter syndrome legal, 40.9% of mothers with fetuses with Klinefelter syndrome attempted abortion. In France the rate of pregnancy termination for Klinefelter syndrome declined significantly following the creation of multidisciplinary prenatal diagnosis centers (46.9% before versus 11.6% afterwards). Stringent follow-up is performed by geneticists, pediatricians, and endocrinologists if the parents decide to continue the pregnancy [8].
This study had an important limitation. The data were collected from a single institution and were retrospectively investigated. Thus, the study results are subject to the problems inherent in that study design; namely, selection bias and lack of the parents' medical and chromosomal information.
In our study, the incidence of Klinefelter syndrome in high-risk patients was 0.24%, which is higher than in previous studies. As the mother's age increases, the incidence of Klinefelter syndrome is thought to also increase. Data from more institutions are needed to determine a more precise incidence rate of Klinefelter syndrome. Also, more aggressive testing before and after birth is needed for proper management and treatment of patients with Klinefelter syndrome.
References
1. Jacobs PA, Strong JA. A case of human intersexuality having a possible XXY sex-determining mechanism. Nature. 1959. 183:302–303.
2. Klinefelter HF Jr, Reifenstein EC Jr, Albright F Jr. Syndrome characterized by gynecomastia, aspermatogenesis without Leydigism, increased excretion of follicle stimulating hormone. J Clin Endocrinol. 1942. 2:615–627.
3. Moore KL. Sex reversal in newborn babies. Lancet. 1959. 1:217–219.
4. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003. 88:622–626.
5. Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 1991. 87:81–83.
6. Philip J, Lundsteen C, Owen D, Hirschhorn K. The frequency of chromosome aberrations in tall men with special reference to 47, XYY and 47, XXY. Am J Hum Genet. 1976. 28:404–411.
7. Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter's syndrome. Lancet. 2004. 364:273–283.
8. Gruchy N, Vialard F, Decamp M, Choiset A, Rossi A, Le Meur N, et al. Pregnancy outcomes in 188 French cases of prenatally diagnosed Klinefelter syndrome. Hum Reprod. 2011. 26:2570–2575.
9. Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C. Male infertility: role of genetic background. Reprod Biomed Online. 2007. 14:734–745.
10. De Souza E, Morris JK. EUROCAT Working Group. Case-control analysis of paternal age and trisomic anomalies. Arch Dis Child. 2010. 95:893–897.
11. Ferguson-Smith MA, Yates JR. Maternal age specific rates for chromosome aberrations and factors influencing them: report of a collaborative european study on 52 965 amniocenteses. Prenat Diagn. 1984. 4 Spec No:5–44.
12. Morris JK, Alberman E, Scott C, Jacobs P. Is the prevalence of Klinefelter syndrome increasing? Eur J Hum Genet. 2008. 16:163–170.
13. Amory JK, Anawalt BD, Paulsen CA, Bremner WJ. Klinefelter's syndrome. Lancet. 2000. 356:333–335.
14. Foresta C, Galeazzi C, Bettella A, Stella M, Scandellari C. High incidence of sperm sex chromosomes aneuploidies in two patients with Klinefelter's syndrome. J Clin Endocrinol Metab. 1998. 83:203–205.
15. Kamischke A, Baumgardt A, Horst J, Nieschlag E. Clinical and diagnostic features of patients with suspected Klinefelter syndrome. J Androl. 2003. 24:41–48.
16. Van Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, et al. Cytogenetics of infertile men. Hum Reprod. 1996. 11:Suppl 4. 1–24.
17. Damani MN, Mittal R, Oates RD. Testicular tissue extraction in a young male with 47,XXY Klinefelter's syndrome: potential strategy for preservation of fertility. Fertil Steril. 2001. 76:1054–1056.
18. Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997. 17:363–368.
19. Ratcliffe SG. The sexual development of boys with the chromosome constitution 47,XXY (Klinefelter's syndrome). Clin Endocrinol Metab. 1982. 11:703–716.
20. Ross JL, Samango-Sprouse C, Lahlou N, Kowal K, Elder FF, Zinn A. Early androgen deficiency in infants and young boys with 47,XXY Klinefelter syndrome. Horm Res. 2005. 64:39–45.
21. Simpson JL, de la Cruz F, Swerdloff RS, Samango-Sprouse C, Skakkebaek NE, Graham JM Jr, et al. Klinefelter syndrome: expanding the phenotype and identifying new research directions. Genet Med. 2003. 5:460–468.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download