Abstract
Purpose
To review the feasibility of laparoscopic ureteroneocystostomy with extracorporeal eversion of the ureteral end in various distal ureteral lesions.
Materials and Methods
We conducted a retrospective review of 5 laparoscopic procedures of ureteroneocystostomy with extracorporeal eversion of the ureteral end. Of these, 4 patients (range, 45 to 54 years) had distal ureter stricture or obstruction after gynecological surgeries for endometriosis or a large uterine myoma. One patient (male, 67 years) had low-grade distal ureter cancer. The laparoscopic procedure was combined with cystoscopic insertion of a ureteral stent and extracorporeal eversion of the ureter through the 10-mm port on the affected side.
Results
The laparoscopic ureteral reimplantations with and without a psoas hitch in patients with distal ureteral lesions was successful in all patients. The mean operation time was 137 minutes (range, 104 to 228 minutes). Two patients underwent additional psoas hitch. In all patients, short-term success was confirmed by voiding cystourethrography and intravenous pyelography conducted 3 months after the operation. The mean follow-up of the entire group was 12 months (range, 3 to 30 months). We noted no major or minor complications over the follow-up period.
Conclusions
The technique of laparoscopic ureteroneocystostomy for benign or malignant ureteral strictures continues to evolve. Surgeons should be versatile with various options and technical nuances when dealing with these cases. Simple modifications of laparoscopic ureteroneocystostomy with extracorporeal eversion of the ureteral end, nonreflux extravesical anastomosis, and simultaneous cystoscopy will be crucial to the ease of performance and a successful outcome.
Laparoscopic ureteroneocystostomies have recently been performed as a definite treatment for ureteral stenosis (either neoplastic or iatrogenic). The techniques used to treat these lesions may or may not include the simultaneous performance of a distal ureterectomy. The surgical principles of maintaining appropriate ureteral continuity and performing watertight and tension-free vesicoureteral anastomosis must be respected [1].
Ureteral injuries are not rare complications after any difficult abdominopelvic surgical procedure, including gynecologic, obstetric, general surgical, and urologic surgery. Distal ureteral injuries are more commonly diagnosed after or during laparoscopic pelvic surgery, which is now a widely used technique for pelvic surgery. As a result, there is a growing need to repair these complications without additional incisions. Moreover, recent reports have described laparoscopic ureteric segmental resection with ureteral reimplantations for the minimally invasive management of low-grade upper urinary tract transitional cell carcinoma [2,3].
For ureteral strictures, various treatment options are available. However, the localization, length, and etiology of the stricture affect the choice of technique. Short distal ureteric defects can be managed by end-to-end anastomosis or ureteroneocystostomy. Occasionally, longer obstructed ureter segments may require complex techniques, such as a psoas hitch or Boari-flap [4-6].
To the best of our knowledge, this is the first case series experience of laparoscopic ureteroneocystostomy with extracorporeal eversion of the ureteral end with a reasonable follow-up. In addition, the role of laparoscopy in the management of distal ureteral stricture due to iatrogenic injury and an early stage of malignancy is evaluated, with a focus on feasibility, safety, and short-term outcome.
Between December 2008 and January 2012, a single surgeon (SWP) performed five laparoscopic ureteroneocystostomies with or without a psoas hitch for benign or malignant lesions in the distal ureter. The etiology of the ureteral reimplantations was iatrogenic injury after hysterectomy in two patients, infiltrative endometriosis in two patients, and low-grade distal ureter cancer in one patient (Table 1). The status of the strictures or lesion was evaluated preoperatively with a combination of intravenous pyelography or antegrade pyelography and computed tomography scans. Preoperatively, two cases presented with complete obstruction. Two patients had a ureteral stent, whereas two patients had percutaneous nephrostomy tubes placed before surgery. We retrospectively collected information on patient demographics and performed follow-up intravenous pyelography and voiding cystourethrography studies 3 months after surgery.
Under general anesthesia, the patient was positioned in the lithotomy position for simultaneous cystoscopy and laparoscopy. A pneumoperitoneum was obtained by Veress needle insufflations with CO2 gas. A 12-mm trocar was placed at the umbilicus for the camera, and two 5-mm trocars were placed in the right and left lower quadrants. An additional 10-mm port on the affected side was added for extracorporeal eversion of the ureteral end. Simultaneously, a cystoscope was prepared for intraoperative ureteral catheter insertion and for confirming the neoureteral orifice. All surgical steps were performed purely laparoscopically via transperitoneal access, except for extracorporeal ureteral eversion.
The procedure began by incising the retroperitoneum along the line of Toldt and by medializing the colon on the side of the affected ureter. The ureter could be easily identified crossing over the common iliac artery. Once identified, the ureter was carefully dissected down to the level of the stricture. Isolation of the obstructed ureteral segment can occasionally be challenging because of previous inflammation, infection, or scaring. It is essential that care is taken during ureteric dissection to preserve the ureteric blood supply to prevent excessive devascularization. The ureter was transected just superior to the level of the stricture or at a minimum distance from the malignant lesion.
After transecting the ureter above the narrow lesion or safety tumor margin, the ureter was prepared for removal. The lower end of the affected ureter was held with a laparoscopic grasper and was brought out the abdominal wall from the ipsilateral 10-mm port. The desufflated abdomen helped to reduce the amount of ureteric mobilization required for extracorporeal eversion. Ureteral intussusception to produce a nipple was easily performed outside the abdomen (Fig. 1).
Excellent mobilization of the bladder is essential for the creation of a tension-free anastomosis. Division of the contralateral superior vesicle pedicle may allow additional bladder mobilization. We identified an area on the bladder on the cranial aspect of the posterior bladder wall where an approximately 3-cm detrusor incision for the neoureteral orifice was made with a 1-cm mucosal incision. After several stitches for vesicoureteral anastomosis, a suitably sized double-J ureteral stent was passed with the aid of a guidewire through the simultaneous cystoscope in a retrograde manner into the proximal ureter and kidney. After the remaining suturing for the mucosa-to-mucosa anastomosis was completed with 4-0 polyglactin (Vicryl) interrupted sutures, normal saline was instilled to confirm that the anastomosis was watertight (Fig. 1).
Two cases had a ureteral defect that was too long to direct vesicoureteral anastomosis. These patients underwent additional dissection of the contralateral perivesical plane and a psoas hitch.
Detrusorrhaphy was created as in the open Lich-Gregoir technique. The detrusor was incised by cautery down to the level of the mucosa, which should remain intact. Apposition of the muscle fibers and perivesical tissue above the anastomosed ureter was undertaken, producing the second plane of detrusorrhaphy while creating a 2-cm long submucosal tunnel. Closure proceeded distally to proximally. Care was taken to not close the tunnel too tightly and to not kink the ureter, both of which can result in ureteral obstruction. The peritoneal incision was then closed with a running absorbable suture (Fig. 1).
The demographics of the five patients are listed in Table 1. Laparoscopic ureteroneocystostomy was completed in all patients without the need for conversion. The mean stricture length was 2.5 cm. Right ureteroneocystostomy was performed in three patients and on the left in two patients. A psoas hitch was performed on two patients. Mean operation time was 137 minutes (range, 104 to 228 minutes), and average blood loss was 100 mL (range, 50 to 250 mL). No patients were transfused. Mean length of hospital stay was 7 days. In all patients, the Foley catheter was removed at 7 days postoperatively after a cystogram showed no evidence of anastomotic leakage.
The double-J ureteral stent was removed 1 month after surgery. Subsequent intravenous pyelography and voiding cystourethrography revealed no evidence of stricture or reflux 3 months after surgery. The mean length of follow-up of the entire group was 12 months (range, 3 to 30 months). We noted no major or minor complications over the follow-up period.
The complication of ureteral injury in laparoscopic surgery in gynecology was first presented in 1974 [7]. The incidence of iatrogenic ureteral injuries in laparoscopy has increased since then owing to the increasing complexity of laparoscopic surgeries and retroperitoneal dissection. Gynecologic pelvic surgery is now the most common cause of iatrogenic ureteral injury. The estimated incidence is from 0.1% to 2.5%. Unfortunately, approximately half of these cases are not identified or corrected during surgery [4]. Fewer injuries to the ureter are immediately identified after laparoscopy. Therefore, during laparoscopy, a high index of suspicion for ureteral injury is required.
Delayed diagnosis of ureteral injury is most often achieved by computed tomography, antegrade ureterography, retrograde ureterography, or intravenous pyelography. Repair of these delayed-recognition injuries is more complex. First-line minimally invasive management is an immediate attempt at placement of a double-J ureteral stent, but this is possible in only 20% of patients [8]. When stent placement is possible, some authors have reported an ultimate success rate as high as 73% without the need for additional surgery [9]. Failure to place a stent usually prompts corrective surgery. Different laparoscopic techniques have been described for the treatment of distal ureteral stenoses, ranging from resection of the ureteral lesion and direct uretero-ureteral anastomosis to reimplantation by means of the psoas hitch or Boari flap [1,6,10].
Recent reports have described laparoscopic ureteric segmental resection with ureteral reimplantations for the minimally invasive management of low-grade upper urinary tract transitional cell carcinoma [2,3]. Recently, robot-assisted laparoscopic surgery for distal ureteral malignancy was reported [11]. Those authors suggested that minimally invasive surgery for malignancy is a feasible, safe, and effective option for distal ureteral malignancy. Because midureteral and distal-ureteral tumors are not amenable to endoscopic resection, distal ureterectomy with ureteral reimplantation is a treatment option. When the ureteral length is insufficient for direct reimplantation, additional length can be gained with either a psoas hitch or a Boari flap.
The performance of the antireflux technique in ureteroneocystostomy is probably the most troublesome part of this procedure. Although some authors did not perform it [2,12,13], most of the reports preferred some antireflux mechanism [1,6,10,14,15]. Various submucosal tunneling techniques have been introduced. It is convenient to inject serum or normal saline under the mucosa through a laparoscope or cystoscope [15]. During the procedure, the tunnel can be partially torn, however, which requires reconstruction. Nonrefluxing anastomosis using a bladder mucosal flap has also been introduced [1,6]. It also requires an additional complex suture. These techniques are not easy to perform without hesitation. Modi et al. [10,14] used a nonrefluxing extravesical Lich-Gregoir technique to prevent vesicoureteric reflux. The authors initially incised the detrusor and bladder mucosa by use of electrocautery, and anastomosed the bladder mucosa and ureteral end by means of a 4-zero interrupted suture. Apposition of the muscle fibers and pericystium above the anastomosed ureter was undertaken, creating a 1- to 2-cm long submucosal tunnel. This extravesical nonrefluxing technique was feasible and produced a low complication rate [5,14,16]. We used the same approach for the antireflux structure, which significantly simplified the surgical procedure.
The eversion of the ureteral end for ureteroneocystostomy reduces postoperative restricture [17-20]. Although ureteral intussusception to produce a nipple is considered an important part of ureteral reimplantation in open surgery, it has never been reported accompanying laparoscopic ureteroneocystostomy. Our series is the first report of extracorporeal eversion of the ureteral end during laparoscopic ureteroneocystostomy. Extracorporeal eversion of the ureteral end for laparoscopic ureteroneocystostomy could make the procedure easier with fewer postoperative complications.
One of the fundamental principles of a ureteroneocystostomy is to achieve tension-free anastomosis. We had to appropriately move the ureter, preserving the periureteral fat for the purpose of preventing ischemia, and also appropriately move the bladder. Occasionally, for achievement of greater bladder movement, it was necessary to dissect the contralateral superior vesical artery, which allowed us to fill the ureteral defect up to 5 cm without the need to resort to a psoas hitch or Boari flap.
Fergany et al. [21] reported their experience with laparoscopic Boari flap ureteroneocystostomy in a porcine model. Six female pigs were used to perform a laparoscopic bladder flap ureteroneocystostomy. Robotics may make this procedure easier with better vision and dexterity during suturing [22]. Many authors have reported that a bladder Boari-flap or psoas-hitch procedure can be successfully used to bridge ureteral defects [1,6,13,14,23,24].
An additional or established port or a percutaneous needle was used to place a ureteral stent in previous reports [1,2,6,25]. A no-stent technique was also introduced [14]. Several authors have recommended that placement of the new ureteral orifice in the bladder and the double-J stent in the affected ureter be monitored by simultaneous cystoscopy and laparoscopy [13,15]. In all our cases, a double-J stent was inserted through simultaneous cystoscopy before completing the ureteric reimplantation. We believe that placing a double-J stent by use of an intraoperative cystoscope is fast and easy and allows us to check the watertight suture and shape of the neoureteral orifice.
The ureter is most vulnerable laparoscopically at the infundibulopelvic ligament and uterine artery. Despite prophylactic ureteral stenting before surgery to prevent iatrogenic ureteral injury, all clinicians should still promptly recognize any injuries so that immediate correction can be carried out.
Antireflux and no-stricture techniques should be considered for laparoscopic ureteroneocystostomy. However, there are several obstacles to performing purely laparoscopic surgery. We recommend an extravesical reimplantation with detrusorrhaphy for antireflux vesicoureteral anastomosis. In addition, ureteral eversion might reduce the recurrence of stricture after surgery. In our cases, an established port on the affected side was used for eversion of the ureteral end. Simple modifications with our current technique for laparoscopic ureteroneocystostomy will be crucial for ease of performance and a successful outcome.
Figures and Tables
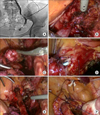 | FIG. 1Preparation of the distal ureter and vesicoureteral anastomosis. (A) Antegrade ureterography showing a 3-cm stricture in the right distal ureter. (B) The obstructed ureteral segment was dissected to the lower end. (C) Ureteral intussusception to produce a nipple was performed outside the abdomen. (D) A double-J ureteral stent was passed with the aid of a guidewire simultaneously through the cystoscope in a retrograde manner. (E) Normal saline was instilled to confirm that the anastomosis was watertight after the remaining mucosa-to-mucosa anastomosis. (F) Detrusorrhaphy created a second plane for antireflux. |
References
1. Simmons MN, Gill IS, Fergany AF, Kaouk JH, Desai MM. Technical modifications to laparoscopic Boari flap. Urology. 2007. 69:175–180.
2. Roupret M, Harmon JD, Sanderson KM, Barret E, Cathelineau X, Vallancien G, et al. Laparoscopic distal ureterectomy and anastomosis for management of low-risk upper urinary tract transitional cell carcinoma: preliminary results. BJU Int. 2007. 99:623–627.
3. Gerber E, Dinlenc CZ, Wagner JR. Laparoscopic distal ureterectomy for low grade transitional cell carcinoma. J Urol. 2003. 169:2295.
4. Park JH, Park JW, Song K, Jo MK. Ureteral injury in gynecologic surgery: a 5-year review in a community hospital. Korean J Urol. 2012. 53:120–125.
5. Azioni G, Bracale U, Scala A, Capobianco F, Barone M, Rosati M, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010. 19:292–297.
6. Rassweiler JJ, Gozen AS, Erdogru T, Sugiono M, Teber D. Ureteral reimplantation for management of ureteral strictures: a retrospective comparison of laparoscopic and open techniques. Eur Urol. 2007. 51:512–522.
7. Stengel JN, Felderman ES, Zamora D. Ureteral injury complication of laparoscopic sterilization. Urology. 1974. 4:341–342.
8. Ghali AM, El Malik EM, Ibrahim AI, Ismail G, Rashid M. Ureteric injuries: diagnosis, management, and outcome. J Trauma. 1999. 46:150–158.
9. Dowling RA, Corriere JN Jr, Sandler CM. Iatrogenic ureteral injury. J Urol. 1986. 135:912–915.
10. Modi P, Goel R, Dodiya S. Laparoscopic ureteroneocystostomy for distal ureteral injuries. Urology. 2005. 66:751–753.
11. Hemal AK, Nayyar R, Gupta NP, Dorairajan LN. Experience with robot assisted laparoscopic surgery for upper and lower benign and malignant ureteral pathologies. Urology. 2010. 76:1387–1393.
12. Gao J, Dong J, Xu A, Wang W, Shi L, Guo G, et al. A simplified technique for laparoscopic ureteroneocystostomy without ureteral nipple or submucosal tunneling. J Endourol. 2007. 21:1505–1508.
13. Nerli RB, Reddy MR, Ravish IR, Amarkhed SS. Laparoscopic bladder (Boari) flap ureteroneocystostomy. J Multidiscip Healthc. 2008. 1:15–16.
14. Modi P, Gupta R, Rizvi SJ. Laparoscopic ureteroneocystostomy and psoas hitch for post-hysterectomy ureterovaginal fistula. J Urol. 2008. 180:615–617.
15. Chung H, Jeong BC, Kim HH. Laparoscopic ureteroneocystostomy with vesicopsoas hitch: nonrefluxing ureteral reimplantation using cystoscopy-assisted submucosal tunneling. J Endourol. 2006. 20:632–638.
16. Lakshmanan Y, Fung LC. Laparoscopic extravesicular ureteral reimplantation for vesicoureteral reflux: recent technical advances. J Endourol. 2000. 14:589–593.
17. Hill JE, Dodson AI Jr, Hooper JW Jr. Experimental ureteroneocystostomy using nipple anastomosis technique. J Urol. 1955. 74:596–599.
18. Urquhart-Hay D, McIlwaine J, Stephenson CB. The use of a ureteric nipple alone for reflux prevention. Br J Urol. 1977. 49:375–377.
19. Elem B. Use of a ureteric nipple in the surgical management of bilharzial ureteric strictures. Br J Urol. 1985. 57:137–140.
20. Abou-Elela A, Torky M, Salah E, Morsy A, Elsherbiny M. Inverted ureteral nipple as antireflux technique in surgical management of bilharzial ureteral strictures. Urology. 2010. 76:983–987.
21. Fergany A, Gill IS, Abdel-Samee A, Kaouk J, Meraney A, Sung G. Laparoscopic bladder flap ureteral reimplantation: survival porcine study. J Urol. 2001. 166:1920–1923.
22. Schimpf MO, Wagner JR. Robot-assisted laparoscopic Boari flap ureteral reimplantation. J Endourol. 2008. 22:2691–2694.
23. Fugita OE, Dinlenc C, Kavoussi L. The laparoscopic Boari flap. J Urol. 2001. 166:51–53.
24. Basiri A, Karami H, Mehrabi S, Javaherforooshzadeh A. Laparoscopic distal ureterectomy and Boari flap ureteroneocystostomy for a low-grade distal ureteral tumor. Urol J. 2008. 5:120–122.
25. Mitre AI, Lestingi JF, Arap MA, Lucon AM, Srougi M. Totally laparoscopic ureteroneocystostomy with intracorporeal tailoring for primary obstructive megaureter. Clinics (Sao Paulo). 2011. 66:177–179.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download