Abstract
Purpose
To compare the stone clearance rate, efficiency quotient (EQ), and early complications of shock wave lithotripsy (SWL) and percutaneous nephrolithotomy (PCNL) for solitary lower-pole renal stones measuring 15 to 20 mm.
Materials and Methods
This was a retrospective matched-pair analysis of 142 patients (78 in the SWL and 64 in the PCNL group). Preoperative imaging was done by use of noncontrast computed tomography (CT kidney, ureter, and bladder [KUB]), intravenous urogram, or plain X-ray and ultrasound KUB to assess the largest dimension of the stones. Only patients with radiopaque stones were included. The stone-free rates were assessed with plain X-ray and ultrasound at 4 weeks. Data were analyzed by use of SPSS ver. 19.
Results
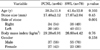
The patients' demographic profiles (age, body mass index) and the stone sizes were comparable in the two groups. The mean stone size was 17.4±2.12 in the PCNL group compared with 17.67±2.04 in the SWL group (p=0.45). At 4 weeks, 83% of patients undergoing PCNL were stone-free compared with 51% in the SWL group (p<0.001). The EQ for the PCNL group was 76% compared with 44% for the SWL group (p<0.001). Ancillary procedures were required by 9% of patients in the PCNL group compared with 15% in the SWL group. The complication rate was 19% in both groups. The SWL complications were minor.
The optimal management of lower-pole calculi has been under debate, and the ideal treatment remains controversial [1]. Shock wave lithotripsy (SWL) was introduced in the early 1980s for the management of renal stones. The success of SWL is dependent on many factors, including stone size, location, composition, stone burden, body habitus, and the availability of a lithotripter for stone clearance [1,2]. Lower calyceal anatomy has been investigated with conflicting results, with some reports favoring its role in predicting stone clearance and others challenging its impact on the clearance rate [3,4].
SWL has high patient acceptance owing to its safety profile; however, significant ancillary procedures and re-treatment rates are some of the drawbacks of this procedure [1]. On the other hand, although percutaneous nephrolithotomy (PCNL) has a higher success rate, and low re-treatment and ancillary procedure rates, it is associated with anesthesia-related risks, hemorrhage, and sepsis [5]. The European Association of Urology (EAU) 2011 guidelines recommend SWL for stones less than 20 mm and PCNL for stones more than 20 mm in size; however, they also recommend using PCNL preferentially because the efficacy of SWL is limited in the setting of lower calyceal stones [6].
The optimal management of lower calyceal stones continues to be a significant problem. SWL is regarded as the first choice for stones <15 mm, and PCNL is considered for stones >20 mm. A stone size of 15-20 mm is considered a grey zone. The present study was designed to compare these two modalities in terms of stone-free rates and complications for lower-pole solitary calculi in the "grey zone" (15-20 mm in size).
This was a retrospective study done over a period of 6 years from January 2005 to December 2010. The radiological and clinical data of all adult patients who were treated for isolated, solitary, radiopaque (assessed on plain film of X-ray kidney, ureter, and bladder [KUB] or scout film of computed tomography [CT] KUB), lower-pole renal stones between 15 and 20 mm in the largest dimension by either SWL or PCNL were included in the study. Preoperative imaging (CT KUB, intravenous urogram [IVU], X-ray KUB, and/or ultrasound) was used to assess the largest dimension of the stone. Patients were counseled concerning the pros and cons of SWL and PCNL for a given stone.
All patients in the lithotripsy arm were treated as outpatients by use of a Siemens Modularis lithotriptor (Siemens AG, Medical Solutions, Erlangen, Germany) under intravenous sedo-analgesia by using pethidine or midazolam with diclofenac in weight-adjusted dosages. The decision for pre-SWL stent placement was according to the preference and decision of the treating consultants; no definite protocol was followed. All patients were treated by a single operator with nearly 20 years' experience in SWL under the supervision of an admitting consultant. The rate of administration of shock waves was 60-90/min. All cases were followed up weekly with X-ray and ultrasound to assess fragmentation and clearance. Follow-up SWL sessions were planned at least 1 week after the prior session. SWL was considered to have failed if no fragmentation was noted after 3 sessions.
PCNL was performed as a single-stage, single-puncture procedure in the operating room with the patient under general anesthesia in the prone position. None of the patients required prior percutaneous nephrostomy. The procedure was in the standard fashion. All patients were followed up 4 weeks after the last session of SWL or PCNL.
The stone-free rate was defined as no evidence of stone fragments on a plain X-ray KUB or ultrasound during follow-up. Clinically insignificant residual fragments were defined as stone fragments of 3 mm or less in largest dimension without any symptoms (pain, fever, hematuria, etc.) or evidence of obstruction. Patients with stone fragments >3 mm were considered as treatment failures. The efficiency quotient (EQ) for both groups was determined by using the standard formula:
The primary outcomes measured were the stone-free rate, the EQ, the need for ancillary procedures (all additional procedures including postprocedure stenting), and the complications incurred.
All clinical and radiological data were collected and analyzed by use of the SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). Qualitative variables were compared by using Pearson's chi-square test and 2×2 test, and Student's t-test was used to compare quantitative variables. p<0.05 was considered statistically significant in all analyses.
A total of 2,612 patients underwent SWL for renal calculi during the study period, of whom 78 (3%) fulfilled the inclusion criteria and were included in the study. Similarly, a total of 585 PCNL procedures were done, of which 64 patients (11%) met the inclusion criteria. The demographic profiles of the two groups were comparable (Table 1). The mean stone size was 17.4±2.12 in the PCNL group compared with 17.67±2.04 in the SWL group (p=0.45). The primary modality for the preoperative diagnosis of stones was CT in both groups.
All 78 patients in the SWL arm had a mean of number of 3.04±1.3 (range, 2 to 6) sessions for stone disintegration and clearance, and the median number of shock waves given was 6,750±3,500 (range, 3,000 to 18,000). In most patients, combined fluoroscopy and ultrasound were used for stone localization. In more than half of the patients, a size 26 Fr Amplatz sheath was used for the placement of the nephroscope, whereas tubeless PCNL was done in 14% of the patients.
The proportion of patients who were stone-free after 4 weeks was significantly higher in the PCNL group than in the SWL group (83% vs. 51%, p<0.001). Nine percent of patients in the PCNL group required ancillary procedures (mainly SWL) compared with 11.5% in the SWL group; however, this difference was not statistically significant. The predominant ancillary procedures in this subset of patients were placement of ureteric stents and ureteroscopy (Table 2). The overall outcome in terms of the EQ was also significantly better for PCNL than for SWL (76% vs.44%, p<0.001).
The overall complication rate was low in both groups (Table 3). Bleeding complications were more common in the PCNL group, and three patients required blood transfusions (modified Clavien Grade [MCG] 2) and one patient required selective angio-embolization of a pseudo-aneurysm of the lower polar vessel (MCG 3a). Eight patients developed steinstrasse after SWL requiring lithotripsy of the lead fragment, ureteroscopy, or placement of a ureteric stent (MCG 3a/3b). One patient developed a renal hematoma after SWL that was managed conservatively. There was no mortality in either treatment arm during the admission or follow-up period.
We performed a matched pair analysis of SWL and PCNL for patients who had solitary lower calyceal renal stones without any other associated abnormalities. SWL has been the mainstay of treatment of urinary tract calculi because of its wide availability, ease of use, efficacy, and safety since its introduction some 30 years ago [7].
Regarding lower-pole calculi, SWL is generally recommended for stones <15 mm, whereas PCNL is considered for stones >20 mm in the largest dimension [6]. A stone size of 15-20 mm in this location is considered to be a grey zone, with urologists favoring either of the two procedures.
Various modalities have been used for the treatment of stones in this location. These include PCNL, SWL, and ureteroscopy (retrograde intra renal surgery [RIRS]). Following SWL, the stone clearance rate for lower-pole calculi is reported to range from 37% to 96% [8-11].
It is generally agreed that the decreased efficacy of SWL is due to retention of stone fragments rather than to disintegration of the stone. This poor result of SWL is because of the dependent position of the inferior calyx and its relationship to the renal pelvis, especially for an acutely angled inferior calyx and narrower infundibulum with a longer calyx [3,4,12].
Yuruk et al. [5] in a randomized trial determined the natural course of asymptomatic lower-pole calculi and compared the deleterious effects of SWL, PCNL, and observation on the kidney. They showed that PCNL achieved a higher stone-free rate and less renal scarring than did SWL.
Observation alone for asymptomatic renal stones is not recommended because >50% of cases of asymptomatic calyceal stones will require some intervention within 5 years owing to obstruction and pain, increasing size of the stone, or associated infection [13,14]. Lingeman et al. [15] in their meta-analysis compared both SWL and PCNL for renal stone management and found a higher stone-free rate (90%) with PCNL than with SWL (59%) and also showed that the clearance rate of stone fragments was worse for lower calyces than for middle and upper calyces.
Albala et al. [9] in a multicenter randomized controlled trial compared PCNL with SWL for symptomatic lower-pole kidney stones sized 30 mm or less. The postoperative stone-free rates after 3 months of follow-up were 95% for percutaneous removal compared with 37% for lithotripsy. The overall EQ was 28% for SWL and 86% for PCNL. For stones ranging in size from 11 to 20 mm specifically, the EQ was 17% and 88%, respectively. They concluded that for PCNL, the stone-free rate was independent of stone burden. Our stone-free rate and EQ were similar to the results reported by Albala et al. [9] for stones between 11 and 20 mm.
The complication rates in both groups were similar; however, a large proportion of patients in the SWL group had MCG 3 complications because of interventions as the result of steinstrasse. Our study showed the incidence of steinstrasse following SWL to be about 10% despite placement of a ureteral stent. Our previously reported study showed an incidence of 7% following SWL for renal stones of all sizes and locations [16]. We concluded that ureteral stents decrease the acute presentations of patients with steinstrasse but have no effect on the need for intervention. There is no clear explanation for the higher incidence of steinstrasse in the current SWL cohort despite the moderate stone bulk.
Chiong et al. [17] in a randomized controlled study of mechanical percussion, dieresis, and inversion therapy (PDI) showed that at 3 months patients with SWL alone had a stone clearance rate of 35.4% compared with 62.5% for the SWL+PDI group. In another randomized controlled trial, Pace et al. [18] reported a substantially higher stone-free rate with PDI therapy than in those treated with observation alone. None of these methods was used in any of our patients, which could have impacted the stone clearance.
The EAU guidelines recommend PCNL if 3 to 5 SWL treatment sessions have failed [6]. Yuruk et al. [19] demonstrated that PCNL is more difficult with a prolonged operative time and fluoroscopic screening time following a failed SWL session because of the tissue effects of SWL (e.g., scarring) and scattered stone fragments in the pelvicaliceal system leading to the need for more punctures and the possibility of leaving residual fragments behind.
Recently, an increasing number of papers are emerging on the efficacy of flexible ureteroscopy for lower-pole renal stones. The indications for RIRS include patients who are morbidly obese, patients who have a bleeding disorder, patients with stones resistant to SWL, patients with a complicated intra-renal anatomy, and as a salvage procedure after failed SWL [20].
Pearle et al. [21] in a randomized controlled trial compared SWL with RIRS using ureteroscopy for lower calyceal stones sized 10 mm or less. A stone-free rate of 65% was achieved for SWL compared with 72% for RIRS at 3 months, which was not statistically significant.
SWL has variable results, and many factors, including the patient's body habitus, the stone location in the collecting system, stone composition, and the operator's experience, influence stone clearance. Kanao et al. [22] developed a preoperative nomogram for predicting the stone-free rate after SWL, which can be a useful adjunct for counseling patients before SWL. They showed that stone size, location, and numbers are significant predictors of the stone-free rate after SWL.
The main limitations of our study were the small sample size and the retrospective nature of the study. Although we tried to exclude patients with anatomical abnormalities (pelvi-ureteric junction obstruction, calyceal diverticulum, and infundibular stenosis) on the basis of available preprocedure investigations, these are better addressed by IVU than by other imaging modalities such as a CT scan, which was the predominant mode of imaging in our study. The use of Hounsfield measurements to determine the density of stones was also not available for all patients; therefore, density was not assessed.
We compared only the clinical outcome and complications between the two modalities but did not address the cost-effectiveness of the procedures, which should be considered in the context of evaluating efficacy. However, the assessment of economics in stone management is not easy [23], because it involves not only the direct procedure and admission-related costs but also the indirect cost of loss of work productivity. Moreover, this estimate will differ and vary depending upon the geographical location and health care system of the various countries.
Owing to the scant data on stone composition, this important aspect was also not addressed in our study. However, stone composition might have direct implications for stone disintegration in SWL patients.
A large majority of our patients were followed with X-ray KUB or ultrasound following the procedure, which may have limited our ability to detect residual stones. Denstedt et al. [24] showed that a plain film can miss up to 35% of residual stones. A CT scan is claimed to be the most sensitive method for detecting residual stones [25,26].
Stone clearance from lower-pole solitary stones sized 15 to 20 mm at their greatest diameter following SWL is poor. These calculi can be better managed with percutaneous surgery owing to its high degree of efficacy and acceptably low morbidity. Large prospective trials comparing the two modalities are needed to further confirm this notion for stones sized 15 to 20 mm in their largest dimension.
References
1. Ather MH, Abid F, Akhtar S, Khawaja K. Stone clearance in lower pole nephrolithiasis after extra corporeal shock wave lithotripsy - the controversy continues. BMC Urol. 2003. 3:1.
2. Gurocak S, Kupeli B, Acar C, Tan MO, Karaoglan U, Bozkirli I. The impact of pelvicaliceal features on problematic lower pole stone clearance in different age groups. Int Urol Nephrol. 2008. 40:31–37.
3. Elbahnasy AM, Shalhav AL, Hoenig DM, Elashry OM, Smith DS, McDougall EM, et al. Lower caliceal stone clearance after shock wave lithotripsy or ureteroscopy: the impact of lower pole radiographic anatomy. J Urol. 1998. 159:676–682.
4. Sampaio FJ, Aragao AH. Inferior pole collecting system anatomy: its probable role in extracorporeal shock wave lithotripsy. J Urol. 1992. 147:322–324.
5. Yuruk E, Binbay M, Sari E, Akman T, Altinyay E, Baykal M, et al. A prospective, randomized trial of management for asymptomatic lower pole calculi. J Urol. 2010. 183:1424–1428.
6. Turk C, Knoll T, Petrik A, Sarica K, Straub M, Seitz C. Guidelines on Urolithiasis. Update March 2011 [Internet]. 2011. cited 2011 Jan 25. Arnhem: European Association of Urology;Available from: http://www.uroweb.org/gls/pdf/18_Urolithiasis.pdf.
7. Chaussy C, Fuchs G. Extracorporeal lithotripsy in the treatment of renal lithiasis. 5 years' experience. J Urol (Paris). 1986. 92:339–343.
8. Ruggera L, Beltrami P, Ballario R, Cavalleri S, Cazzoletti L, Artibani W. Impact of anatomical pielocaliceal topography in the treatment of renal lower calyces stones with extracorporeal shock wave lithotripsy. Int J Urol. 2005. 12:525–532.
9. Albala DM, Assimos DG, Clayman RV, Denstedt JD, Grasso M, Gutierrez-Aceves J, et al. Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis-initial results. J Urol. 2001. 166:2072–2080.
10. Elbahnasy AM, Clayman RV, Shalhav AL, Hoenig DM, Chandhoke P, Lingeman JE, et al. Lower-pole caliceal stone clearance after shockwave lithotripsy, percutaneous nephrolithotomy, and flexible ureteroscopy: impact of radiographic spatial anatomy. J Endourol. 1998. 12:113–119.
11. Juan YS, Chuang SM, Wu WJ, Shen JT, Wang CJ, Huang CH. Impact of lower pole anatomy on stone clearance after shock wave lithotripsy. Kaohsiung J Med Sci. 2005. 21:358–364.
12. Sampaio FJ, Aragao AH. Limitations of extracorporeal shockwave lithotripsy for lower caliceal stones: anatomic insight. J Endourol. 1994. 8:241–247.
13. Glowacki LS, Beecroft ML, Cook RJ, Pahl D, Churchill DN. The natural history of asymptomatic urolithiasis. J Urol. 1992. 147:319–321.
14. Inci K, Sahin A, Islamoglu E, Eren MT, Bakkaloglu M, Ozen H. Prospective long-term followup of patients with asymptomatic lower pole caliceal stones. J Urol. 2007. 177:2189–2192.
15. Lingeman JE, Siegel YI, Steele B, Nyhuis AW, Woods JR. Management of lower pole nephrolithiasis: a critical analysis. J Urol. 1994. 151:663–667.
16. Ather MH, Shrestha B, Mehmood A. Does ureteral stenting prior to shock wave lithotripsy influence the need for intervention in steinstrasse and related complications? Urol Int. 2009. 83:222–225.
17. Chiong E, Hwee ST, Kay LM, Liang S, Kamaraj R, Esuvaranathan K. Randomized controlled study of mechanical percussion, diuresis, and inversion therapy to assist passage of lower pole renal calculi after shock wave lithotripsy. Urology. 2005. 65:1070–1074.
18. Pace KT, Tariq N, Dyer SJ, Weir MJ, D'A Honey RJ. Mechanical percussion, inversion and diuresis for residual lower pole fragments after shock wave lithotripsy: a prospective, single blind, randomized controlled trial. J Urol. 2001. 166:2065–2071.
19. Yuruk E, Tefekli A, Sari E, Karadag MA, Tepeler A, Binbay M, et al. Does previous extracorporeal shock wave lithotripsy affect the performance and outcome of percutaneous nephrolithotomy? J Urol. 2009. 181:663–667.
20. Preminger GM. Management of lower pole renal calculi: shock wave lithotripsy versus percutaneous nephrolithotomy versus flexible ureteroscopy. Urol Res. 2006. 34:108–111.
21. Pearle MS, Lingeman JE, Leveillee R, Kuo R, Preminger GM, Nadler RB, et al. Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2008. 179:5 Suppl. S69–S73.
22. Kanao K, Nakashima J, Nakagawa K, Asakura H, Miyajima A, Oya M, et al. Preoperative nomograms for predicting stone-free rate after extracorporeal shock wave lithotripsy. J Urol. 2006. 176(4 Pt 1):1453–1456.
23. Koo V, Young M, Thompson T, Duggan B. Cost-effectiveness and efficiency of shockwave lithotripsy vs flexible ureteroscopic holmium: yttrium-aluminium-garnet laser lithotripsy in the treatment of lower pole renal calculi. BJU Int. 2011. 108:1913–1916.
24. Denstedt JD, Clayman RV, Picus DD. Comparison of endoscopic and radiological residual fragment rate following percutaneous nephrolithotripsy. J Urol. 1991. 145:703–705.
25. Gaucher O, Cormier L, Deneuville M, Regent D, Mangin P, Hubert J. Which is the best performing imaging method for demonstrating residual renal calculi. Prog Urol. 1998. 8:493–501.
26. Lehtoranta K, Mankinen P, Taari K, Rannikko S, Lehtonen T, Salo J. Residual stones after percutaneous nephrolithotomy; sensitivities of different imaging methods in renal stone detection. Ann Chir Gynaecol. 1995. 84:43–49.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download