Abstract
Purpose
This study aimed to determine the effects of the long-term use of dehydroepiandrosterone sulfate (DHEAS) on rat prostates and testes as well as on serum testosterone and DHEAS levels.
Materials and Methods
Thirty male rats aged 4 to 5 months were studied. A DHEAS suspension of 5 mg/kg per rat was administered orally to the 15 rats in the experimental group 5 times a week, whereas saline was administered concurrently to the 15 rats in the control group. Intracardiac blood samples were drawn to determine hormone levels, and histological samples of prostate and testes were evaluated under light microscopy.
Results
At the end of the 6-month study period, histological examinations performed on prostate preparations showed that the atrophy score of the experimental group was significantly lower than the scores of the sham and control groups (p<0.001 and p<0.001, respectively). The serum total testosterone and DHEAS levels of the rats in the study group were significantly increased (p<0.001).
Conclusions
In our study, we determined that the long-term use of DHEAS does not have any detrimental effects on the prostate or the testis; on the contrary, it protects the prostate from atrophy, which is imperative for the continuation of fertility as well as for increasing serum testosterone and DHEAS levels.
As society ages, many people have tried to prevent aging and its effects by using different drugs, such as antioxidants and hormones. Recently, trends have shifted to the use of testosterone and major secretory products of the human adrenal cortex, dehydroepiandrosterone (DHEA) and its sulfate ester dehydroepiandrosterone sulfate (DHEAS), as antiaging medicines. DHEA and DHEAS act as sources of active androgens in some of the androgen-responsive tissues and as precursors for androstenedione in peripheral tissues [1]. The secretion of DHEA in humans and in some nonhuman primates follows an age-related pattern. At birth, DHEA is present at high levels in the serum, because it is produced by the fetal adrenal glands. During the first years of life, the serum level of DHEA decreases and it remains almost undetectable until 6 to 10 years of age. Peak DHEA concentrations are reached during early adulthood. At 70 to 80 years of age, the concentrations of DHEA steadily decline to become only 10% to 20% of those of young adults [2].
Although these molecules were identified more than 70 years ago, their exact physiological roles remain to be defined. Some of the beneficial effects of DHEAS include an antidiabetic effect, the prevention of osteoporosis, the modulation of the immune system, antiobesity actions, the modulation of the central nervous system, anticarcinogenesis actions, and the prevention of atherosclerosis [3,4]. However, the effects of this new drug are not fully understood, especially regarding androgen-dependent organs. This study aimed to demonstrate the effects of the chronic use of DHEAS on the histology of androgen-dependent organs, such as the prostate and the testis, and on total serum testosterone and DHEAS levels in normal aging rats.
Thirty Wistar Kyoto male rats aged 4 to 5 months, which can be considered adult, and weighing between 300 and 400 g were used for this study [5]. The animals were placed in standard rat cages in a controlled environment at the Cukurova University Medical Sciences Experimental Research and Implementation Centre. Each cage accommodated a maximum of seven or eight rats, and all activities within the scope of the study were performed with the approval of the ethics committee, under the supervision of a veterinarian and in compliance with the provisions of the Strasbourg Universal Declaration of Animal Rights of 1986. All animals were kept in the same room with a constant mean temperature of 21℃±2℃ and light from 0700 to 1900. The rats were fed water and pelleted food that was specifically prepared for rodents. Veterinary and experienced personnel in the center removed any associated animal waste and supplied water and feed to the animals.
The animals were divided into three groups. Group 1 included 8 rats that received 5 mL of saline solution orally once daily 5 days each week for 6 months. In group 2, 7 animals were treated as shams. Group 3 included 15 rats that received 5 mg/kg DHEAS solution orally once daily 5 days each week for the same time period as the other groups. This dose was administered according to the protocol used in a similar study in which the standard DHEAS supplementation dose in humans was increased by a factor of 10 to account for the higher hepatic metabolic activity of rats compared with humans [6]. There was no sign of hypogonadism or male climacterium in the rats, because they were of an adult age group.
At the end of the 6-month experimental period, the rats were anesthetized intraperitoneally with a combination of 6 mg/kg 2% xylazine hydrochloride (Rompun, Bayer, Leverkusen, Germany) and 75 mg/kg ketamine hydrochloride (Ketalar, Pfizer, New York, NY, USA). The skin, subcutaneous tissue, fascia, and peritoneum of the rats were opened with 3-cm incisions on the lower abdomen. Afterwards, the testes and prostate of each rat were located and carefully dissected from the surrounding tissues. The incision was then elongated through the thorax, and a 3-mL intracardiac blood sample was withdrawn for biochemical analysis.
Total testosterone and DHEAS levels were measured by immunoassay techniques by use of an E170 immunoassay analyzer (Roche, Mannheim, Germany).
The prostate glands and testes were fixed in 10% formalin and stained with hematoxylin and eosin. Two different paraffin blocks were prepared from each testis and prostate. Three randomized power fields at ×200 magnification were analyzed by a single pathologist blinded to the treatment that the animal received. The histological sections were analyzed and quantified with respect to the degrees of atrophy and hyperplasia. Furthermore, the stroma gland ratio and papillary component were graded on a semiquantitative scale of 1 to 3 as defined previously [6]. The histologies of the testes were analyzed according to the Johnsen testicular biopsy score count [7].
Statistical analyses were performed by using the Mann-Whitney U test for the histological score counts and the serum analysis. Both chi-square and Fisher's exact tests were used for the comparison of the categorical variables. In the multiple analysis, Bonferroni corrections (p<0.05/n, where n is the comparison number) were performed. A value of p<0.018 was considered significant.
The results of the biochemical analyses showed serum DHEAS levels to be 8.0±1.4, 7.3±1.4, and 280.6±71 ng/mL and testosterone levels to be 1.9±0.4, 1.9±0.2, and 3.9±0.6 ng/mL in the control, sham, and experimental groups, respectively. These values were significantly higher in the experimental group than in the control and sham groups (p=0.001) (Table 1). However, no statistically significant differences in prostate or testis weights were observed between the groups (p>0.05).
Histological examinations performed on the testis preparations revealed that spermatogenesis was complete in seminiferous tubules and that there were no score differences between the control, sham, and experimental groups on the basis of the Johnsen scoring protocol (p>0.05).
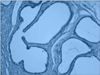
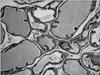
However, histological examinations performed on the prostate preparations showed that the atrophy score of the experimental group was significantly lower than the scores of the control and sham groups (p<0.001 and p<0.001, respectively) (Table 2; Figs. 1, 2). The stroma-gland ratio, the papillary component, and hyperplasia were analyzed as categorical variables. The percentages of the stroma-gland ratio, the papillary component, and hyperplasia were not significantly different in the experimental group compared with the sham group (p=0.09, p=0.6, and p=0.2, respectively), whereas the percentage of atrophy was significantly lower in the experimental group (p<0.05).
DHEA and DHEAS are major secretory steroids secreted by the adrenal gland. Principally, all of the DHEA present in male plasma originates from the adrenal cortex, and the daily production amount ranges from 10 to 30 mg in humans [8]. Whereas DHEA is secreted in a pulsatile and circadian manner, DHEAS does not display such variations in level; it is secreted at an average level. For this reason, DHEAS serum levels are used for both clinical and laboratory studies [9].
Because of the difficulties involved in human experiments, animal model experiments are performed to arrive at clear definitions of the biological functions of DHEA and DHEAS in the body and to evaluate the histological responses to treatment. In our study, we examined the effects of chronic use of DHEAS, which is currently administered as an anti-aging agent, on the testis and prostate as androgen-dependent organs. When we compared the experimental group, which was subjected to chronic DHEAS administration over a 6-month period, with the control and sham groups, the serum DHEAS level of the experimental group was determined to be 35 and 38 times higher, respectively; the total testosterone level of the experimental group was determined to be 2 times higher than that of the two other groups (p=0.001). The serum levels of these two substances were determined to be comparable in the control and sham groups, which is concordant with the literature [6-8].
When we evaluated the prostate tissue, we observed that the proportion of cells displaying atrophy was significantly lower in the experimental group than in the control and sham groups (p=0.0001 and p=0.0001, respectively). In the first publication on this issue by Zizzi et al. [10], signs of prostatic atrophy were shown in the experimental group. By contrast, Rhoden et al. [6] reported no signs of atrophy in either the study or the control group. No variations were observed between the prostate cells of the three groups in terms of atypia in the direction of hypertrophy, hyperplasia, or malignancy; dysplasia; and prostatic intraepithelial neoplasia. Although utmost care must be exercised when applying the data obtained in animals to humans, our results suggest that DHEA protects the volume of the prostate, an important organ for fertility, but does not cause hyperplasia. However, DHEA must be used carefully, especially in individuals who are at risk in terms of malignancy, because DHEA administration is known to increase testosterone levels.
In the literature search we performed on testis and long-term androgen treatment, we found no studies on the histological effects of androgen replacement on testes in healthy humans. However, when testes weights were measured in a study that evaluated long-term DHEA administration in rats, no significant differences were identified between the experimental and control groups [6,8]. Likewise, in the histopathological examinations of the testes, we found that spermatogenesis was complete and that there were no significant differences between the groups in terms of Johnsen scoring and testes weights (p>0.05).
Information on the possible side effects caused by use of DHEA as a medicine is very limited. The biggest concern of clinicians regarding the oral use of DHEA is the possibility of causing prostate cancer or triggering its development. Indeed, the increased testosterone level observed during DHEA treatment suggests that these concerns are not groundless. Additionally, concerns intensified after Morgentaler [11] identified prostate cancer in biopsies of 11 of 77 hypogonadic male patients receiving testosterone treatment whose rectal examination results and prostate-specific antigen (PSA) levels were normal. Morley et al. [12] showed that average PSA levels increased significantly at 3 months after testosterone treatment. Similarly, Tenover [13] also determined an average increase of 0.6 µg/L in PSA levels after 3 months of testosterone treatment.
In contrast, many studies in the literature show that androgen replacement does not induce prostate cancer in males with normal prostates. Horton [14] and Mac Donald [15] separately reported that less than 1% of the testosterone present in the plasma is obtained from DHEA. Similarly, in the Reiter et al. [16] study, which used oral administration of 50 mg DHEA, the authors did not find any statistically significant changes in PSA levels, prostate volume, or urinary residue after urination. Similar data suggesting that DHEA had no effect at all on urination symptoms were also presented by Flynn et al. [17] after a 3-month observation period. Additionally, Rhoden et al. [6] reported an increase in plasma testosterone levels without any change in prostate weight or histology in a study of DHEA administration in rats. Also, the authors suggested that long-term use does not induce changes towards malignancy [18,19].
Regarding the side effects of androgen replacement treatment, not many studies in the literature have been conducted on healthy human testes, and there is no consensus other than the contraindications for the use of DHEA in prostate cancer patients. Many questions await answers in further studies. Although some of our results were statistically significant, it should be kept in mind that this study had some limitations. The tissue and preoperative biochemical levels of testosterone could not be measured owing to limited funds, because this study was supported by the Academic Research Projects Unit of our university. Also, this study should be considered a pilot study because it was done with a small sample size of animals and minimal parameters to generalize the evidence of the role of DHEAS.
Today, as the aging population increases worldwide, many people are turning toward anti-aging treatments; hormone replacement treatments are also used for this purpose. DHEA and DHEAS are probably the most popular hormones used for these treatments. Although such treatments are popular and are thought to be useful, the literature does not provide much information regarding their side effects. Thus, we sought to assess the effects of DHEAS on androgen-dependent organs (i.e., the prostate and testes). Our study revealed that long-term DHEAS use does not cause adverse effects on the prostate and testis in rats; by contrast, it protects the prostate, an important organ in terms of sustaining fertility, from atrophy. Additionally, we also determined that chronic DHEAS use increased serum testosterone and DHEAS levels. We believe that our findings may prove valuable for future studies of the effects of DHEA and DHEAS.
ACKNOWLEDGMENTS
The authors thank the staff of the Cukurova University Medical Sciences Experimental Research and Implementation Centre for the animal care and also thank Associate Professor Gulsah Seydaoglu for the statistical analysis.
This study was supported by the Academic Research Projects Unit of the University of Cukurova, grant number TF2005LTP7.
References
1. Hornsby PJ. DHEA: a biologist's perspective. J Am Geriatr Soc. 1997. 45:1395–1401.
2. Allolio B, Arlt W. DHEA treatment: myth or reality? Trends Endocrinol Metab. 2002. 13:288–294.
3. Herbert J. The age of dehydroepiandrosterone. Lancet. 1995. 345:1193–1194.
4. Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994. 78:1360–1367.
5. Zanato VF, Martins MP, Anselmo-Franci JA, Petenusci SO, Lamano-Carvalho TL. Sexual development of male Wistar rats. Braz J Med Biol Res. 1994. 27:1273–1280.
6. Rhoden EL, Gobbi D, Rhoden CR, Menti E, Roehe AN, Hartmann A, et al. Effects of chronic administration of dehydroepiandrosterone on serum testosterone levels and prostatic tissue in rats. J Urol. 2003. 170:2101–2103.
7. Johnsen SG. Testicular biopsy score count: a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970. 1:2–25.
8. Gobbi D, Rhoden EL, Menti E, Lulhier F, Rhoden C. Effects of the chronic use of dehydroepiandrosterone (DHEA) on testicular weight and spermatogenesis: experimental study in rats. Int Urol Nephrol. 2003. 35:119–122.
9. Hinson JP, Raven PW. DHEA deficiency syndrome: a new term for old age? J Endocrinol. 1999. 163:1–5.
10. Zizzi T, Nunnery M, Cason Z, Tucci M, Benghuzzi H. The effects of dehydroepiandrosterone and dehydroepiandrosterone sulfate on the reproductive and vital organs of male rats. Biomed Sci Instrum. 1999. 35:279–284.
11. Morgentaler A, Bruning CO 3rd, DeWolf WC. Occult prostate cancer in men with low serum testosterone levels. JAMA. 1996. 276:1904–1906.
12. Morley JE, Perry HM 3rd, Kaiser FE, Kraenzle D, Jensen J, Houston K, et al. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. 1993. 41:149–152.
13. Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992. 75:1092–1098.
14. Horton RJ. Grayhack JT, Wilson JD, Scherbenske MJ, editors. Androgen hormones and prehormones in young and elderly men. Benign Prostatic Hyperplasia. Proceedings of Workshop Sponsored by Kidney Disease and Urology Program of the NIAMDD. 1976. Washington, DC: US Government Printing Office;183–188.
15. Mac Donald PC. Grayhack JT, Wilson JD, Scherbenske MJ, editors. Origin of estrogen in man. Benign Prostatic Hyperplasia. Proceedings of Workshop Sponsored by Kidney Disease and Urology Program of the NIAMDD. 1976. Washington, DC: US Government Printing Office;191–192.
16. Reiter WJ, Pycha A, Schatzl G, Klingler HC, Mark I, Auterith A, et al. Serum dehydroepiandrosterone sulfate concentrations in men with erectile dysfunction. Urology. 2000. 55:755–758.
17. Flynn MA, Weaver-Osterholtz D, Sharpe-Timms KL, Allen S, Krause G. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab. 1999. 84:1527–1533.
18. Severi G, Morris HA, MacInnis RJ, English DR, Tilley W, Hopper JL, et al. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006. 15:86–91.
19. Gould DC, Kirby RS. Testosterone replacement therapy for late onset hypogonadism: what is the risk of inducing prostate cancer? Prostate Cancer Prostatic Dis. 2006. 9:14–18.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download