INTRODUCTION
Cutaneous ureterostomy (CU) is the simplest and safest among all methods of permanent urinary diversions. However, after cystectomy for bladder cancer, an ileal conduit is considered the standard form of urinary diversion because CU is associated with a significant risk of stomal stenosis [1]. Nevertheless, if CU can be successfully achieved without a tube, late complications are reduced [2], and the procedure appears to be as good as the ileal conduit procedure. Various attempts have been made to decrease the frequency of complications [2-6]. A high catheter-free rate of 89.8% in 59 renal units (RUs) was reported by the introduction of a new surgical stabilization step for the abdominal wall tunnel [7]. In general, it was assumed that the renal functions were preserved after the establishment of tubeless CU, so that there would be only a very low possibility that ureteral stent catheters might be needed [2]. However, no reports have examined the occurrence of hydronephrosis after the establishment of tubeless CU, and there are no diagnostic criteria to evaluate hydronephrosis in CU. Therefore, the main purpose of this study was to provide follow-up data for hydronephrosis after the establishment of tubeless CU. We investigated our definition of the tubeless condition in CU and our indications for catheter insertion in the management of tubeless CU.
MATERIALS AND METHODS
We retrospectively reviewed the medical charts and follow-up data for 30 patients who underwent CU between October 2005 and March 2011 at our hospital. Of these patients, 28 patients (54 RUs) with both the establishment of tubeless CU at 3 months after surgery and at least 12 months of follow-up were enrolled in this study. The underlying disease was bladder cancer in 26 patients and bladder cancer after retroperitoneoscopy-assisted laparoscopic nephroureterectomy with a cuff for unilateral renal pelvic cancer in 2 patients. The patients comprised 24 men and 4 women with an average age of 71.1±7.7 years (range, 54 to 87 years). The patients' mean body mass index (BMI) was 22.3±3.0 kg/m2 (range, 16.7 to 29.0 kg/m2). The pathologic stages of bladder cancers were 0 in 3 patients (10.7%), I in 5 patients (17.9%), II in 14 patients (50.0%), III in 5 patients (17.9%), and IV in one patient (3.6%).
The CU was constructed after complete cystectomy with or without urethrectomy. In 26 patients (92.9%), both ureters were used to construct the CU with unilateral stomal creation (on the right side in 19 and on the left side in 7 patients). In the other two patients, one ureter was used to construct the CU (on the right side in one and on the left side in one patient), because these two patients had undergone retroperitoneoscopy-assisted laparoscopic nephroureterectomy with cuff for left or right renal pelvic cancer. All 28 patients successfully underwent unilateral stomal creation (on the right side in 20 and on the left side in 8 patients). The surgical procedure by Straffon et al. [8] was used in these patients for creating the course of the ureters. The ureters were brought through in a completely extraperitoneal manner in all patients, and the stoma was created with the Toyoda method [4]. After the surgery, a 6-Fr single-J stent was placed in the renal pelvis through the stoma. In all cases, the single-J stents were exchanged every 4 weeks and were removed 3 months after the surgery, because the stomal conditions were unstable and obstructive in the early phases after the surgery [9,10].
The four-grade system was used to evaluate the hydronephrosis [11]. Our definition of the tubeless condition in the CU was as follows: 1) the catheter stent is not placed in the renal pelvis through the stoma, 2) the grade of hydronephrosis is less than 3, and 3) the kidney is functioning. Indications for catheter insertion after the establishment of tubeless CU were as follows: 1) difficulty in curing acute pyelonephritis by drug treatments, 2) flank pain due to hydronephrosis, or 3) increase of the grade of hydronephrosis. Student's t-test was used for statistical analysis. Significance was defined as p<0.05.
RESULTS
The follow-up period was from 12 to 78 months (average, 40.5±22.1 months). When our study ended in March 2012, a total of 20 patients were alive without disease, 3 patients had died of their disease, 1 patient was alive with his disease, 1 patient was alive with another type of cancer, and 3 patients had died of other diseases.
Before the surgery, hydronephrosis of grades 1 and 2, respectively, was present in two RUs (3.7%) owing to ureteral obstruction by bladder cancer. After the removal of the single-J stents (3 months after the surgery) in all 54 RUs that achieved a tubeless condition, 21 (38.9%) had no hydronephrosis. Hydronephrosis of grades 1, 2, and 3 without the need for intervention was present in 13 (24.1%), 18 (33.3%), and 2 RUs (3.7%), respectively. Three months after surgery, the grade of hydronephrosis in all 54 RUs was less than 3. A catheter insertion was performed in one RU (1.9%) owing to both acute pyelonephritis and the persistence of grade 2 hydronephrosis 5 months after surgery. Six months after surgery, of 53 RUs (98.1%) that achieved a tubeless condition, 48 (88.9%) had no hydronephrosis. Hydronephrosis of grades 1 and 2 without the need for intervention was present in two (3.7%) and one RU (1.9%), respectively, and the grade 2 hydronephrosis had persisted for 61 months. The serum creatinine (s-Cre) levels of this patient were 1.35 and 1.40 mg/dL before the surgery and 5 years after the surgery, respectively, which suggests that renal function had been stable in this patient for 5 years. The two patients with grade 1 hydronephrosis 6 months after surgery are referred to as case 1 and case 2, respectively.
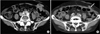
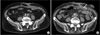
Six RUs (11.1%) of 5 patients who developed grade increases of hydronephrosis or developed acute pyelonephritis that was difficult to cure by drug treatments fulfilled the indications for catheter insertion. The causes were retroperitoneal lymph node (RPLN) recurrence of bladder cancer or gall bladder cancer in two patients whose grade of unilateral hydronephrosis increased from 0 to 3 at 17 and 15 months after surgery, respectively; acute pyelonephritis and the persistence of grade 2 hydronephrosis in one patient at 5 months after surgery; parastomal hernia in one patient (case 1); and bilateral ureteral kinking owing to excessive decrease of BMI in one patient (case 2). At the end of the follow-up period, catheter insertion was performed in 4 RUs, and the other 2 RUs were followed up without intervention because of advanced age (more than 80 years old) and no symptoms due to hydronephrosis. However, these two RUs gradually became atrophic. The average time to the detection of the grade increase of hydronephrosis or the insertion of a ureteral stent catheter was 11.5±5.5 months (range, 5 to 17 months). Parastomal hernia (case 1) and excessive decrease of BMI (case 2) were unusual causes of the grade increase of hydronephrosis after the construction of tubeless CU. Case 1 complained of an abdominal wall protrusion in the stomal area 9 months after surgery. Computerized tomography revealed a sac protruding through an area of the abdominal wall near the stoma (Fig. 1A). We had constructed the abdominal wall tunnel for the ureters in the extreme lateral area of the rectus muscle in this case, which was assumed to be an inappropriate surgical technique for creating the hiatus for stomal positioning (Fig. 1B). The parastomal hernia was asymptomatic and did not interfere with the use of an appliance. Although the left kidney did not show hydronephrosis, a grade 1 hydronephrosis was present in the right kidney 6 months after surgery, which gradually increased to grade 2, and the right renal function gradually worsened. Because the patient was 81 years old and did not complain of any symptoms due to the hydronephrosis, the patient was followed up conservatively. Case 2 lost his appetite after surgery, resulting in a BMI decrease from 29.0 to 22.0 kg/m2 over 15 months. Although right grade 1 hydronephrosis was improved 9 months after surgery, bilateral grade 2 hydronephrosis appeared to be due to obstruction of the ureters at the level of the posterior sheath of the rectus muscle (Fig. 2). The insertion of ureteral catheter stents was tried with both antegrade and retrograde approaches. However, because a guidewire could not pass through the obstructive position of the ureters, bilateral nephrostomies were constructed percutaneously. The patient refused additional treatments for the ureteral obstructions, and the 14-Fr nephrostomy catheters were exchanged every 4 weeks.
We were able to obtain follow-up data for s-Cre levels in 28, 13, and 9 cases 1 year, 3 years, and 5 years after surgery, respectively. S-Cre levels were 0.94±0.24 mg/dL, 0.99±0.32 mg/dL (p=0.452), 0.99±0.24 mg/dL (p=0.495), and 1.00±0.28 mg/dL (p=0.509) before the surgery and 1, 3, and 5 years after the surgery, respectively. No significant differences were found among s-Cre levels during the 5-year follow-up period. It appeared, therefore, that renal function had been preserved for about 5 years after the establishment of tubeless CU.
DISCUSSION
We presented follow-up data on hydronephrosis after the establishment of tubeless CU by using our definition of the tubeless condition and our indications for catheter insertion. Six of 54 RUs (11.1%) fulfilled our indications for catheter insertion. The catheter insertion was performed in 4 RUs (7.4%). Another 2 RUs (3.7%), which had not received ureteral stent catheters, became gradually atrophic. Except for these 2 RUs, renal function was preserved in the other 52 RUs. These data suggest that our definition of the tubeless condition and our indications for catheter insertion would be useful for the evaluation and management of tubeless CU.
The ureters were brought through in a completely extraperitoneal manner while creating the course of the ureters. The contralateral ureter was brought to the stomal side behind the mesosigmoid to construct the CU with a unilateral and parallel stoma. Therefore, the contralateral ureter became obstructive by the RPLN recurrence in two of five patients who fulfilled our indications for catheter insertion. Except for these two cases, the ureters became obstructed by complications in the stomal portion. Four of 54 RUs (7.4%) required catheter insertion because of stomal complications after the establishment of tubeless CU.
In general, parastomal hernia has not been observed as a complication of CU. In another study, parastomal hernia was observed in 4 of 272 patients (1.5%) who were treated by CU [10]. To decrease the frequency of stomal stenosis, we constructed a larger abdominal tunnel for the ureters than that described in the general procedure for the CU [7]. Therefore, it seems that the same mechanisms might be responsible for the occurrence of parastomal hernia after the construction of the tubeless CU and ileal conduit. One study showed a 22% incidence of parastomal hernia when the stoma was placed lateral to the rectus muscle, compared to 3% when the stoma was brought through the muscle belly [12]. The pararectal area is probably the weakest area owing to the vessels perforating the fascias. The position of the stoma was constructed in the extreme lateral area of the rectus muscle in our parastomal case, indicating that inappropriate surgical techniques would be definite predisposing factors for parastomal hernia.
In case 2, neither a guidewire nor a 5-Fr ureteral stent was able to pass through the ureters at the level of the posterior sheath of the rectus muscle with either an antegrade or a retrograde approach. Stomal revision would have been required to confirm the cause of the ureteral obstruction and to treat it, but the patient refused the additional surgical treatments after the construction of the bilateral nephrostomy. It was estimated that the tension put on the ureters at the level of the posterior sheath of the rectus muscle by the bodily constitution change with excessive decrease of BMI resulted in the ureteral kinking.
CONCLUSIONS
After the establishment of tubeless CU, renal functions were preserved for about 5 years under management by use of our definition of the tubeless condition and our indications for catheter insertion. Our results suggest that our definition of the tubeless condition and our indications for catheter insertion would be useful for the evaluation and management of tubeless CU.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download