Abstract
Purpose
We evaluated clinical characteristics, sperm retrieval rates, and birth rates in a relatively large number of infertile patients with Y chromosome microdeletions.
Materials and Methods
We retrospectively reviewed clinical data from 213 patients with nonobstructive azoospermia (NOA) and 76 patients with oligoasthenoteratozoospermia (OATS) who were tested for Y chromosome microdeletion from March 2004 to June 2011.
Results
Of the 289 patients, 110 patients presented with Y chromosome microdeletion and 179 patients presented with no microdeletion. Among the patients with Y chromosome microdeletions, 83/110 (75.4%) were NOA patients and 27/110 (24.5%) were OATS patients. After subdividing the patients with Y chromosome microdeletion, 29 had azoospermia factor (AZF)b-c microdeletion and 81 had AZFc microdeletion. The sperm retrieval rate was similar between patients with Y chromosome microdeletion and those with no microdeletion (26.6% vs. 25.6%, p=0.298) after multiple testicular sperm extraction (TESE). Excluding 53 patients who did not undergo TESE, 30 patients were analyzed. All of the 9 men with AZFb-c microdeletion had a complete absence of sperm despite multiple TESE. However, multiple TESE was successful for 9 of 21 patients with only AZFc microdeletion (p=0.041). Twenty patients with Y chromosome microdeletion gave birth.
Conclusions
In NOA and OATS patients, no significant difference in the sperm retrieval rate was shown between patients with Y chromosome microdeletion and those with no microdeletion. Patients with short Y chromosome microdeletion such as AZFc microdeletion have better prognoses for sperm retrieval and an increased chance of conception than do patients with larger microdeletions such as AZFb-c microdeletion.
In infertile couples, male factors account for almost half of the cases of infertility [1]. These male factors can be ascribed to infection, immunological factors, anatomical malformations, or chemical insult. Besides these factors, genetic abnormalities can also cause male infertility. A constitutional chromosomal abnormality can be found in about 15% of azoospermic men and in 6% of men with oligozoospermia [2-4]. In addition to determining the development of spermatogenesis, the human Y chromosome has an important role [5]. The prevalence of Y chromosome microdeletions is approximately 7%, with a range of 1% to 35%; the range depends on research methods and the selection of patients [6,7]. Microdeletion of the long arm of the Y chromosome is frequently correlated with the failure of spermatogenesis [8,9]. Each region of the Y chromosome microdeletion is known as an azoospermia factor (AZF), including factors a, b, and c. A schematic depiction of the location of the AZF regions in the Y chromosome is shown in Fig. 1. Y chromosome microdeletion most frequently involves the AZFc region (60%), less frequently the AZFb region (16%), and only rarely the AZFa interval (5%). Larger Y chromosome microdeletions involving two or three AZF regions are diagnosed in 14% of cases [7].
Before the development of in vitro fertilization with intracytoplasmic sperm injection (ICSI) and sperm retrieval techniques, such as multiple testicular sperm extraction (TESE), most patients with Y chromosome microdeletions were not able to conceive. However, with the use of ICSI and current sperm extraction technologies, recent studies have reported that successful pregnancies are possible [10-12]. In addition, recent studies have shown that spermatozoa can be retrieved from men with AZFc region microdeletion, thus increasing the chances of conception [13-15]. In the present study, we evaluated the clinical characteristics, sperm retrieval rates, and birth rates of a relatively large number of infertile patients with Y chromosome microdeletions of the AZFb or AZFc region. We also investigated the effects of each type of Y chromosome microdeletion with respect to the chance of retrieving spermatozoa with TESE and birth rates.
The patients were selected from an Institutional Review Board-approved database of cases from March 2004 to June 2011. Infertile patients with nonobstructive azoospermia (NOA) or severe oligoasthenoteratozoospermia (OATS) were enrolled this study. All of the men who presented themselves to our center for evaluation of male factor infertility underwent a thorough history and comprehensive physical evaluation. The initial laboratory evaluation included semen analysis and a hormonal profile (follicle-stimulating hormone [FSH], luteinizing hormone [LH], testosterone, estradiol [E2], sex-hormone binding globulin [SHBG]). On the basis of the results of the semen analysis and hormone profile, the patients were divided into two groups: patients with NOA and patients with severe OATS. Additionally, we offered Y chromosome microdeletion gene screening to all of the patients with NOA and severe OATS. The NOA diagnosis was confirmed by multiple TESE for diagnostic and therapeutic purposes. A retrospective chart review was then performed. Birth information was obtained by telephone with the consent of the patients. For analysis, we divided the two groups according to Y chromosome analysis into the Y chromosome microdeletion group and the no microdeletion group. The "no microdeletion group" referred to patients with NOA or OATS who visited the hospital and received a chromosome microdeletion test and were confirmed to not have any abnormalities. A total of 110 men with Y chromosome microdeletions were then divided into two subgroups. One group included patients with AZFb and AZFc microdeletions (AZFb-c). The other group consisted of patients with AZFc microdeletion. We then evaluated the clinical characteristics, sperm retrieval rates, and birth rates of patients with Y chromosome microdeletion of AZFb and/or AZFc.
Y chromosome analysis was performed by using leukocytes in the peripheral blood sample. Y chromosome microdeletions were confirmed with multiplex polymerase chain reaction (PCR). In the men who were found to have a failure of a sequence-tagged site (STS) on the Y chromosome, single primer pairs were used to confirm the absence of each site in a PCR reaction on multiple occasions.
If no sperm were identified within the pellet, multiple TESEs were performed. These multiple TESE procedures were performed by two surgeons. Multiple TESE procedures were performed with either multiple-site biopsies or with single, large incisions with multiple samplings. All biopsy sample collections included evaluation by inverted microscopy for the presence of viable spermatozoa. The procedures were performed in conjunction with the infertility centers that planned the in vitro fertilization cycles with ICSI or sperm freezing.
The data are presented as the mean±standard deviation. Student's t-test was used for continuous data to evaluate comparisons between the groups. The chi-squared test and Fisher's exact test were used for categorical data. A p-value <0.05 was regarded as statistically significant. The statistical analyses were performed by using the IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA).
Of the 289 men with male factor infertility who underwent screening for Y chromosome microdeletions at our center, Y chromosome microdeletions were detected in 110 patients (38.0%). Of these 110 patients, 83 were classified as having NOA and 27 were classified as having severe OATS. The clinical characteristics of these patients are summarized in Table 1. Mean age, mean FSH, mean LH, mean testosterone, mean E2, and mean SHBG were not significantly different between the no microdeletion patients and the Y chromosome microdeletion patients. With the exception of those subjects who refused the multiple TESE procedure, 30 of the 83 NOA patients with Y chromosome microdeletions underwent multiple TESE procedures. The other 53 did not undergo TESE owing to a previously known diagnostic TESE result from another institution, personal refusal, or loss to follow-up. Of the 30 patients, 9 had AZFb-c microdeletion and 21 had AZFc microdeletion. Multiple TESE successfully retrieved sperm in 8 of 30 patients with Y chromosome microdeletions (26.6%). Multiple TESE had similar success with Y chromosome microdeletion patients and no microdeletion patients (8/30 [26.6%] vs. 21/82 [25.6%], p=0.298). A total of 23/138 patients (16.6%) with no microdeletion achieved live birth, as did 20/82 patients (24.3%) with Y chromosome microdeletions. The difference in the birth rate between the no microdeletion group and the Y chromosome microdeletion group was significant (p=0.007). However, because female infertility factors were not available in these data, this result seems questionable.
The 110 men with Y chromosome microdeletions were divided into two subgroups. One group included patients with AZFb and AZFc microdeletion (AZFb-c). The other group consisted of patients with AZFc microdeletion. The different clinical characteristics of the two groups are shown in Table 2. Among the AZFb-c microdeletion patients, 25 patients (86.2%) had NOA and 4 patients (13.8%) had severe OATS. Of the severe OATS patients with AZFb-c microdeletion, the sperm count range was from motile 2-3 sperm/high power field [HPF] to 0.8×106/mL. Patients with AZFc microdeletion had a higher prevalence of severe OATS (NOA, n=58 [71.6%] vs. OATS, n=23 [28.4%]). In the severe OATS patients with AZFc microdeletion, the sperm count range was from motile 2-3 sperm/HPF to 5.8×106/mL. Among the NOA patients with AZFb-c microdeletions, we failed to retrieve sperm with multiple TESE. In contrast, multiple TESE successfully retrieved sperm in 8 of 21 (38.0%) NOA patients with AZFc microdeletions. Multiple TESE was more likely to succeed in AZFc microdeletion patients than in AZFb-c microdeletion patients (p=0.041). The sperm retrieval rates of each group are also shown in Table 2. Among the AZFc microdeletion patients (n=18, 29.0%), the resulting birth rate was higher than that of the AZFb-c microdeletion patients (n=2, 9.5%). Because no sperm was retrieved with multiple TESE in NOA patients with AZFb-c microdeletion, the birth rate in these patients was zero. In contrast, 2 out of 3 (75%) severe OATS patients with AZFb-c microdeletion were able to conceive. Both patients with AZFb-c microdeletion gave birth through ICSI. In the NOA patients with AZFc microdeletion, 8 patients had a chance to conceive through ICSI. Moreover, in severe OATS patients with AZFc microdeletion, 9 patients gave birth through ICSI and one patient gave birth through natural conception. However, the birth rate did not differ significantly between the AZFb-c microdeletion patients and the AZFc microdeletion patients (p=0.085).
In this study, we observed that the incidence of Y chromosome microdeletions was relatively high compared with previous studies [6,7]. We also observed an association between Y chromosome microdeletions and birth rates with multiple TESE. More spermatozoa were retrieved in patients with AZFc microdeletion with multiple TESE than in patients with AZFb-c microdeletion (8/21 [38.0%] vs. 0/9 [0%], p=0.041). We also observed that severe OATS patients with larger Y chromosome microdeletions such as AZFb-c could conceive with ICSI.
Y chromosome microdeletions have been frequently identified in infertile men with azoospermia or very low sperm concentrations in the ejaculate [6,7]. Our study showed a rate of Y chromosome microdeletions of 110/289 (38.0%) among the patients with NOA and severe OATS. This was a much higher rate than the reported prevalences of Y chromosomal microdeletions of 9.6% to 19.4% at other Asian centers. In other studies, it was argued that these large variations could be due to the selection of patient groups, to ethnic differences, to genetic background, or to the STS marker sets used [16,17]. Additionally, in our study, another factor increasing the incidence of Y chromosomal microdeletion was selection bias, such as the referral pattern. Our institution specializes in assisted reproductive technique, and our screened population might have been more phenotypically severe than men treated at other institutions.
According to Tsujimura et al. [18], among 60 patients with NOA, the spermatozoa retrieval rate for patients with Y chromosome microdeletion was similar to that of patients without Y chromosome microdeletion (33.3% vs. 37.0%). In our study, the sperm retrieval rate was similar between no microdeletion patients and Y chromosome microdeletion patients (21/82 [25.6%] vs. 8/30 [26.6%], p=0.298). The birth rates of patients with Y chromosome microdeletions were significantly different from those in patients with no microdeletion (p=0.007); however, when viewed objectively, this conclusion seems somewhat questionable because data regarding female infertility factors were not available.
The type of Y chromosome microdeletion (AZFa, b, c) has been proposed as a potential prognostic factor for sperm retrieval in men undergoing multiple TESE [11,12,14]. Deletions including and extending beyond the AZFc region (i.e., AZFb-c, AZFa-b-c) have been correlated with the complete absence of testicular spermatozoa, and the presence of an AZFb microdeletion is a significantly adverse prognostic finding for multiple TESE. Although the type of Y chromosome microdeletion is a prognostic factor, large series of patients with microdeletions involving the AZFb region have not been fully characterized in the published literature [6,7,9,11,19,20]. We characterized 110 men with AZFb and/or AZFc microdeletions to evaluate prognostic values for sperm retrieval in these patients on the basis of the specific region of the microdeletion. The association between AZFc microdeletions and spermatogenesis has been demonstrated previously [12,14,19-21]. Clearly, patients with AZFc microdeletions have variable capacities to produce sperm, with some producing no spermatozoa within the seminiferous tubules and others producing a quantity of sperm sufficient to survive epididymal transit and appear in the ejaculate [15]. According to van Golde et al. [14], out of 300 OATS patients, 8 had AZFc microdeletion (2.7%), whereas no AZFa or AZFb deletions were found. In our study, 4 of the OATS patients (5.2%) had AZFb-c microdeletion and 23 (30.2%) had AZFc microdeletion. This study enrolled only patients with severe OATS, which resulted in a higher prevalence of the microdeletion, but an inverse relation between microdeletion length and sperm productivity can be inferred. In NOA patients, we report that 81 men with AZFc microdeletions had a sperm retrieval rate of 38.0%. This result is lower than the data from a large cohort of men with AZFc microdeletion studied by Oates et al. [15], who showed that men with AZFc microdeletions had a 67% sperm retrieval rate. The reason for the lower retrieval rate may be that the selection of patients differed from other institutions or the multiple TESE technique differed from other institutions. Many studies have reported that mature spermatozoa are obtained in approximately 50% of patients with AZFc microdeletions, despite reduced fertilization rates and worse embryo scores after ICSI [22]. We reported that 18/62 men (29.0%) with AZFc microdeletions conceived children: one patient through natural conception and 17 patients through ICSI procedures.
Microdeletions of the AZFb region occur more commonly in conjunction with deletions of the AZFc region [21]. Moreover, with large deletions, including those in the AZFa-b-c and AZFb-c regions, birth rates have not been widely reported. We identified 29 men with AZFb-c microdeletion. Kamp et al. [23] reported that sperm retrieval was unsuccessful in all cases of AZFb microdeletion. Brandell et al. [24] also described seven azoospermic patients with microdeletions, including microdeletions of the entire AZFb region; all of their subjects failed to retrieve spermatozoa or sperm in multiple TESE. Krausz et al. [25] reported on the impact of AZFb microdeletions in infertile men, and their findings have been widely discussed. In essence, their findings indicate that in cases of microdeletions that remove the entire AZFb region, the probability of obtaining testicular spermatozoa for successive ICSI is virtually zero. In our data, we also found that we were unable to retrieve sperm from azoospermic patients with AZFb-c microdeletions. However, two patients with severe OATS with AZFb-c microdeletions had a successful chance of pregnancy through ICSI. It seems that NOA men with deletions of the AZFb-c region have almost no possibility of sperm retrieval with TESE. However, if a patient has OATS with AZFb-c microdeletion, ICSI is a possible treatment option. Although larger Y chromosome microdeletion such as AZFb-c microdeletion has a poor prognosis, it is possible that sperm can be retrieved by multiple TESE.
In many centers, Y chromosome microdeletion analysis is still not performed as a routine measure, and as a result, the findings are used for genetic counseling but are not considered to have prognostic value. However, we emphasize that evaluations of Y chromosome microdeletions in infertile men with NOA and severe OATS should be performed before undertaking assisted reproductive techniques, such as multiple TESE and ICSI. In this study, we showed that a reasonable percentage of couples with Y chromosome microdeletions have a chance to conceive. In NOA and OATS patients, a test for Y chromosome microdeletion not only provides essential information for genetic counseling, but also helps the patients and their physicians make more informed decisions about sperm retrieval.
Nonetheless, our findings might have a limited scope. The high incidence of Y chromosome microdeletions that we observed might reflect selection bias due to referral patterns. Furthermore, we had trouble collecting precise information about past pregnancies or birth history on the basis of recorded information and phone calls, and as a result, some patient information was missing. We also had a limitation in our ability to predict the ultimate sperm production capabilities of the male offspring of men with AZF microdeletion. Because genetic testing data from the male offspring in our study were not available, we could not address the inheritance of the Y chromosome microdeletions.
In NOA and OATS patients, no significant difference in the sperm retrieval rate was shown between the Y chromosome microdeletion group and the no microdeletion group. Even though the group with no Y chromosome microdeletion showed a better birth rate than did the Y chromosome microdeletion group, the difference was not clinically significant. NOA patients with AZFc microdeletion reached successful conception with TESE, whereas the AZFb-c microdeletion patients did not. Severe OATS patients were fully capable of giving assisted birth. Patients with short microdeletions in the Y chromosome such as AZFc microdeletion have better prognoses for sperm retrieval and an increased chance of conception compared with patients with larger microdeletions such as AZFb-c microdeletion. However, this does not mean that AZFb-c microdeletion patients are completely infertile. Two patients in the AZFb-c microdeletion group with OATS were able to conceive with ICSI. The sperm-producing ability is not completely abolished in the AZFb-c microdeletion group. Therefore, role of TESE in the large microdeletion group deserves further evaluation.
Figures and Tables
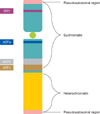 | FIG. 1Schematic depiction the location of azoospermia factor (AZF) regions a, b, and c in the Y chromosome. SRY, sex-determining region of the Y chromosome. |
TABLE 1
Comparisons of clinical outcomes and characteristics in idiopathic cause patients and Y chromosome microdeletion patients

TABLE 2
Comparisons of clinical outcomes and characteristics in patients with AZFb-c microdeletions and solitary AZFc
microdeletions

Values are presented as mean±standard deviation or number (%).
AZFb-c, azoospermic facter b-c; AZFc, azoospermic factor c; FSH, follicle stimulating hormone; LH, luteinizing hormone; SHBH, sex hormone binding globulin; E2, estradiol; NOA, nonobstructive azoospermia; ICSI, intracytoplasmic sperm injection; OATS, oligoasthenoteratozoospermia; HPF, high power field.
References
1. Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod. 1991. 6:811–816.
2. De Braekeleer M, Dao TN. Cytogenetic studies in male infertility: a review. Hum Reprod. 1991. 6:245–250.
3. Nordenson I, Abramsson L, Duchek M. Somatic chromosomal aberrations and male infertility. Hum Hered. 1984. 34:240–245.
4. Bourrouillou G, Bujan L, Calvas P, Colombies P, Mansat A, Pontonnier F. Role and contribution of karyotyping in male infertility. Prog Urol. 1992. 2:189–195.
5. Vogt PH, Edelmann A, Hirschmann P, Kohler MR. The azoospermia factor (AZF) of the human Y chromosome in Yq11: function and analysis in spermatogenesis. Reprod Fertil Dev. 1995. 7:685–693.
6. Foresta C, Moro E, Ferlin A. Y chromosome microdeletions and alterations of spermatogenesis. Endocr Rev. 2001. 22:226–239.
7. Foresta C, Moro E, Ferlin A. Prognostic value of Y deletion analysis: the role of current methods. Hum Reprod. 2001. 16:1543–1547.
8. Simoni M, Bakker E, Krausz C. EAA/EMQN best practice guidelines for molecular diagnosis of y-chromosomal microdeletions: state of the art 2004. Int J Androl. 2004. 27:240–249.
9. Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg M, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet. 1995. 10:383–393.
10. Kent-First MG, Kol S, Muallem A, Ofir R, Manor D, Blazer S, et al. The incidence and possible relevance of Y-linked microdeletions in babies born after intracytoplasmic sperm injection and their infertile fathers. Mol Hum Reprod. 1996. 2:943–950.
11. Oliva R, Margarit E, Ballescá JL, Carrio A, Sanchez A, Mila M, et al. Prevalence of Y chromosome microdeletions in oligospermic and azoospermic candidates for intracytoplasmic sperm injection. Fertil Steril. 1998. 70:506–510.
12. Mulhall JP, Reijo R, Alagappan R, Brown L, Page D, Carson R, et al. Azoospermic men with deletion of the DAZ gene cluster are capable of completing spermatogenesis: fertilization, normal embryonic development and pregnancy occur when retrieved testicular spermatozoa are used for intracytoplasmic sperm injection. Hum Reprod. 1997. 12:503–508.
13. Page DC, Silber S, Brown LG. Men with infertility caused by AZFc deletion can produce sons by intracytoplasmic sperm injection, but are likely to transmit the deletion and infertility. Hum Reprod. 1999. 14:1722–1726.
14. van Golde RJ, Wetzels AM, de Graaf R, Tuerlings JH, Braat DD, Kremer JA. Decreased fertilization rate and embryo quality after ICSI in oligozoospermic men with microdeletions in the azoospermia factor c region of the Y chromosome. Hum Reprod. 2001. 16:289–292.
15. Oates RD, Silber S, Brown LG, Page DC. Clinical characterization of 42 oligospermic or azoospermic men with microdeletion of the AZFc region of the Y chromosome, and of 18 children conceived via ICSI. Hum Reprod. 2002. 17:2813–2824.
16. Kumtepe Y, Beyazyurek C, Cinar C, Ozbey I, Ozkan S, Cetinkaya K, et al. A genetic survey of 1935 Turkish men with severe male factor infertility. Reprod Biomed Online. 2009. 18:465–474.
17. Vutyavanich T, Piromlertamorn W, Sirirungsi W, Sirisukkasem S. Frequency of Y chromosome microdeletions and chromosomal abnormalities in infertile Thai men with oligozoospermia and azoospermia. Asian J Androl. 2007. 9:68–75.
18. Tsujimura A, Matsumiya K, Takao T, Miyagawa Y, Koga M, Takeyama M, et al. Clinical analysis of patients with azoospermia factor deletions by microdissection testicular sperm extraction. Int J Androl. 2004. 27:76–81.
19. Silber SJ, Nagy Z, Devroey P, Tournaye H, Van Steirteghem AC. Distribution of spermatogenesis in the testicles of azoospermic men: the presence or absence of spermatids in the testes of men with germinal failure. Hum Reprod. 1997. 12:2422–2428.
20. Silber SJ, Alagappan R, Brown LG, Page DC. Y chromosome deletions in azoospermic and severely oligozoospermic men undergoing intracytoplasmic sperm injection after testicular sperm extraction. Hum Reprod. 1998. 13:3332–3337.
21. Hopps CV, Mielnik A, Goldstein M, Palermo GD, Rosenwaks Z, Schlegel PN. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003. 18:1660–1665.
22. Saut N, Terriou P, Navarro A, Levy N, Mitchell MJ. The human Y chromosome genes BPY2, CDY1 and DAZ are not essential for sustained fertility. Mol Hum Reprod. 2000. 6:789–793.
23. Kamp C, Huellen K, Fernandes S, Sousa M, Schlegel PN, Mielnik A, et al. High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Mol Hum Reprod. 2001. 7:987–994.
24. Brandell RA, Mielnik A, Liotta D, Ye Z, Veeck LL, Palermo GD, et al. AZFb deletions predict the absence of spermatozoa with testicular sperm extraction: preliminary report of a prognostic genetic test. Hum Reprod. 1998. 13:2812–2815.
25. Krausz C, Quintana-Murci L, McElreavey K. Prognostic value of Y deletion analysis: what is the clinical prognostic value of Y chromosome microdeletion analysis. Hum Reprod. 2000. 15:1431–1434.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download