Abstract
A 49-year-old man presented with an incidentally detected right renal mass on a health examination. The abdominal computed tomography and magnetic resonance imaging showed a 3-cm right renal mass suspected of being a hypovascular tumor, such as papillary renal cell carcinoma, and an osteoblastic metastatic lesion on the right iliac bone. However, we missed a bone lesion at the time of diagnosis. A laparoscopic radical nephrectomy was performed and the final pathology confirmed unclassified renal cell carcinoma. The follow-up imaging studies showed several neck lymph nodes and multiple bone metastases at the lumbar spine, right iliac bone, and left femur. Thirteen cycles of temsirolimus were administered to the patient, but follow-up positron emission tomography showed newly developed liver and left adrenal metastasis and increased bone metastasis. It is important to note that T1a renal cell carcinoma can present with distant metastasis and thus demands scrupulous examination even though the tumor size may be small.
The detection of incidental, small, solid renal tumors (≤4 cm) has steadily increased, mainly because of the widespread use of routine abdominal imaging such as ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) [1]. Most of the small renal tumors can be cured with existing surgical approaches, but there is a small but not insignificant risk of synchronous and metachronous metastatic disease and cancer-associated death. Tumor size alone is not sufficient to distinguish renal cell carcinoma (RCC) with benign behavior from aggressive small RCC [2].
Among the histological subtypes of renal cancer, unclassified RCC is associated with 1.6- to 1.7-times greater RCC-specific mortality than that of patients with clear cell RCC with the same grade and stage characteristics [3].
Herein, we report a case in which the patient presented with a 3-cm sized renal mass with synchronous bone metastasis that was missed at the initial diagnosis and that subsequently progressed rapidly despite radical nephrectomy and adjuvant temsirolimus therapy.
A 49-year-old male presented with an incidentally detected right renal mass on a health examination. The patient had no symptoms or signs associated with the renal mass but presented with mild anemia (hemoglobin, 11.6 g/dl) and elevated alkaline phosphate (170 U/l).
An abdominal CT performed in another hospital showed a 3-cm right renal mass with a homogenous pattern and slight enhancement compared with the common clear cell RCCs (Fig. 1). MRI showed iso-intensity on the T1 weighted image and low signal intensity on the T2 weighted image. Furthermore, an osteoblastic lesion on the right iliac bone was present, but we did not detect the lesion at that time (Fig. 1).
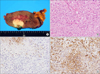
We suspected a hypovascular renal tumor such as papillary RCC and performed laparoscopic radical nephrectomy. The surgery was uneventful and the final pathology confirmed unclassified RCC of Fuhrman grade II, pT1aNxMx (Fig. 2).
Two months after surgery, the patient came to the emergency room with nausea, vomiting, and fever (38.7℃). Laboratory examination showed anemia (hemoglobin, 8.6 g/dl) and elevated alkaline phosphate (404 U/l), lactate dehydrogenase (657 U/l), and C-reactive protein (15.33 mg/dl). The patient's coagulation profiles were prolonged (activated partial thromboplastin time, 60.5 seconds; prothrombin time, 15.3/75/1.20 sec/%/international normalized ratio). To find the fever focus, abdominal and neck CT was performed, which revealed multiple osteoblastic bone metastases and several enlarged neck lymph nodes. Given that we had not previously detected the synchronous right iliac bone metastasis, we initially suspected a hematologic disorder such as multiple myeloma or myelophthisis. To rule these out, a peripheral blood smear was done; it revealed a rouleaux formation. However, bone marrow aspiration and bone biopsy showed metastasis of RCC. Subsequent review of the initial CT and MRI revealed the synchronous bone metastasis.
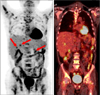
Because the primary histology was the nonclear cell type, temsirolimus was offered to the patient for first-line therapy. Thirteen cycles of temsirolimus (25 mg weekly) was administered to the patient. The latest follow-up positron emission tomography showed newly developed liver and left adrenal metastases and increased bone metastasis (Fig. 3). We recommended interferon plus vinblastine for second-line therapy. The patient refused further chemotherapy and was instead treated conservatively. Finally, the patient died of the disease.
The proportion of small RCC (<4 cm) is increasing because of the growing use of cross-sectional imaging such as CT and MRI [4]. Most patients with small RCC have a good prognosis. However, several studies have shown that 5 to 7% patients with small RCC present with synchronous metastasis and that 5-year, cancer-specific mortality increases in a nonlinear sigmoidal relationship with tumor size [2]. In the present case, the initial CT and MRI were performed at another hospital, so we focused on the right renal mass and did not sufficiently review the other lesions. Also, the European Association of Urology guidelines recommended that bone metastasis is symptomatic at diagnosis; therefore, metastatic evaluation is indicated only if symptoms or laboratory signs are present [5]. However, the patient did not manifest bone metastasis-related symptoms except for a mild elevated alkaline phosphate level; therefore, we overlooked evaluation of bone metastasis. Furthermore, we did not consider the possibility of distant metastasis because the renal mass was only 3 cm in diameter and there were no enlarged lymph nodes.
Recently, the 2004 World Health Organization (WHO) classification divided adult renal epithelial malignant neoplasms into 10 categories [6]. Among them, unclassified RCCs represent 4 to 7% of renal tumors and the features to define this category of disease include composites of recognized types, pure sarcomatoid morphology without recognizable epithelial elements, mucin production, rare mixtures of epithelial and stromal elements, and unrecognizable cell types. Tumors of unrecognizable cell or architecture types or those that are apparent composites of the recognized types are all included in the WHO classification scheme [6]. Microscopic findings of the present study showed no evidence of clear cells, papillary, or acinar structures. This is compatible with the 2004 WHO classification.
Limited reported data suggest the clinical features of unclassified RCC are an aggressive form of RCC, mainly because most reports have been concerned with larger tumors with an advanced stage at presentation [3,7,8]. Zisman et al. [7] reported that unclassified RCC was associated with larger tumors, increased risk of adrenal involvement and involvement of adjacent organs, and increased risk of metastatic involvement of the regional/nonregional lymph nodes and the bones compared with clear cell RCC. In addition, unclassified disease was associated with poor clinical outcomes. These clinical features were also reported by Karakiewicz et al. [3], who also mentioned that unclassified disease was more likely to be associated with Fuhrman grade III or IV, nodal and distant metastasis, and higher mortality than was clear cell disease. In contrast, Crispen et al. [8] reported that advanced clinicopathologic features were related more to unclassified disease, but that overall survival, cancer-specific survival, and distant metastatic-free survival were not different from clear cell histology in a matched analysis. In our case, although we missed a synchronous one-bone metastatic lesion in the initial images, the main renal mass was small (3 cm) and had no regional lymph node involvement or adjacent organ invasion.
Recently, many studies have been published concerning systemically targeted therapies for the treatment of advanced RCCs. For nonclear cell histology, temsirolimus has been shown to be beneficial in terms of overall survival and progression-free survival, regardless of age and risk group [9]. Although the neoadjuvant or adjuvant role of temsirolimus has not been well defined in nonclear cell metastatic disease, Rodriguez Faba et al. [10] reported that neoadjuvant temsirolimus can downstage the T4N2M1 unclassified type RCC to T1bN0M0 and, after imaging, can be negative for recurrence. In our case, unclassified RCC presented extensive bone metastasis 2 months after surgery and, even though we missed a single bone metastatic lesion, temsirolimus was associated with 4.1 months of progression-free survival and 8.4 months of overall survival. In this regard, further studies are needed to clarify the efficacy of temsirolimus before and after cytoreductive nephrectomy.
There are few reports about the T1a unclassified type of RCC with distant metastasis. Although the prevalence of this disease entity is rare, the final pathologic results show unclassified RCC after surgery for a small renal mass. Thus, physicians should review the initial images and pay attention to metastasis, because unclassified RCCs tend to rapidly progress and to carry a poor prognosis.
Figures and Tables
FIG. 1
Pretreatment computed tomography scan showing a 3-cm tumor of the right kidney and an osteoblastic lesion on the right iliac bone (arrow).

FIG. 2
Gross and microscopic findings. (A) The mass was located within the cortex of the kidney and was 3.0×2.0×2.0 cm in size. There was no evidence of cystic change, hemorrhage, or necrosis in the mass. (B) The tumor cells were arranged in solid sheets. The cells were polygonal and the nuclei were moderately hyperchromatic. There was no evidence of clear cells, papillary, or acinar structures (H&E, ×200). (C) Most of the tumor cells were negative for CD10 (×200). (D) The tumor cells displayed diffuse positivity for vimentin (×200).
References
1. Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA. 1999. 281:1628–1631.
2. Klatte T, Patard JJ, de Martino M, Bensalah K, Verhoest G, de la Taille A, et al. Tumor size does not predict risk of metastatic disease or prognosis of small renal cell carcinomas. J Urol. 2008. 179:1719–1726.
3. Karakiewicz PI, Hutterer GC, Trinh QD, Pantuck AJ, Klatte T, Lam JS, et al. Unclassified renal cell carcinoma: an analysis of 85 cases. BJU Int. 2007. 100:802–808.
4. Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001. 166:1611–1623.
5. Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010. 58:398–406.
6. Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006. 49:798–805.
7. Zisman A, Chao DH, Pantuck AJ, Kim HJ, Wieder JA, Figlin RA, et al. Unclassified renal cell carcinoma: clinical features and prognostic impact of a new histological subtype. J Urol. 2002. 168:950–955.
8. Crispen PL, Tabidian MR, Allmer C, Lohse CM, Breau RH, Blute ML, et al. Unclassified renal cell carcinoma: impact on survival following nephrectomy. Urology. 2010. 76:580–586.
9. Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007. 356:2271–2281.
10. Rodriguez Faba O, Breda A, Rosales A, Palou J, Algaba F, Maroto Rey P, et al. Neoadjuvant temsirolimus effectiveness in downstaging advanced non-clear cell renal cell carcinoma. Eur Urol. 2010. 58:307–310.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download