Abstract
The introduction of robot-assisted laparoscopic surgery has facilitated the application of minimally invasive surgical techniques to many complex reconstructive and extirpative procedures. Even early on in their learning experience, robotic surgeons have been able to complete procedures using a minimally invasive approach, but would not have been able to do so using a purely laparoscopic technique. Although the open surgical approach remains the standard of care in the management of large renal tumors presenting with a thrombus within the vena cava, robot-assisted surgery may provide the precision and dexterity necessary to allow for the safe application of minimally invasive techniques to such complex clinical scenarios, perhaps even by relatively novice robotic surgeons. We describe the management of a large renal mass with vena caval thrombus (cT3b), which required complete cross-clamping of the vena cava, with the use of a purely robot-assisted laparoscopic approach.
Renal cell carcinoma (RCC) with evidence of a thrombus extending into the inferior vena cava (IVC) accounts for up to 10% of all cases [1]. The gold standard management of RCC with IVC thrombus remains open radical nephrectomy with caval thrombectomy.
With improved laparoscopic expertise within the field and the introduction of the robotic surgical platform, urologists have been able to use minimally invasive surgical (MIS) techniques to manage complex procedures that traditionally may have required an open approach. Because of the increased precision and dexterity of robotic surgery and the facile learning curve associated with this approach, robotic surgeons have been able to complete procedures using a minimally invasive approach even early on in their learning experience.
We report the purely robot-assisted laparoscopic management of a 12-cm right-sided RCC with IVC tumor thrombus (pT3b), which required complete cross-clamping of the vena cava, by relatively novice robotic surgeons.
A 61-year-old man presented to a community hospital with a 4-month history of fatigue and a 45-kg weight loss. Investigations revealed a serum hemoglobin concentration of 71 g/l, a 12-cm right-sided renal mass, and a 3-cm right-sided adrenal mass. After receiving four units of packed red blood cells, the patient was discharged home and referred to our institution for definitive management.
On initial evaluation at our institution, his vital signs were stable and his body mass index was 43.5 kg/m2. On examination, the patient had a soft, nontender abdomen with a large pannus and no palpable masses. Other results from the physical examination were unremarkable.
Staging imaging showed an enhancing 12.0×10.7-cm right upper pole mass with an adjacent 3.5-cm adrenal nodule and evidence of a possible subhepatic IVC thrombus (Fig. 1). A computed tomography scan of the chest showed no metastases, and the results of a bone scan were also negative. The serum creatinine concentration was 71 µmol/l, liver enzyme concentrations were within normal limits, and the hemoglobin concentration was 82 g/l.
The patient was taken to the operating room for a robotic, possibly open, radical nephrectomy with caval thrombectomy. A vascular surgeon, a hepatobiliary surgeon, and an experienced uro-oncologist were consulted and available for intraoperative assistance.
After all of the ports were placed (Fig. 2) and the robot was docked, the ascending colon was reflected medially, the duodenum was kocherized, and the IVC was exposed. The liver extended beyond the lower pole of the kidney and was sufficiently large enough that a single 5-mm grasper was unable to hold the weight of the liver. Thus, another 5-mm assistant port was placed lateral to the initial 5-mm assistant port to help hold the liver off of the kidney (Fig. 2).
The renal artery was identified and transected, by using a standard laparoscopic stapling device, in the interaortocaval space to minimize handling of the IVC and thrombus-filled right renal vein. The IVC was then dissected circumferentially above and below the right renal vein; two gonadal veins were clipped and transected, which left a 1.5-cm stump on both, and the right adrenal vein and two lumbar veins were also clipped and transected. The left renal vein was dissected circumferentially in the interaortocaval space. Once the kidney was completely mobilized, except for its lateral attachments to the abdominal wall, the fourth robotic arm was docked and used to provide lateral retraction of the kidney.
A laparoscopic ultrasound probe was used to visualize the tumor thrombus (Fig. 3). After it was confirmed that the thrombus was not adherent to the IVC wall, that there was no bland thrombus below the tumor thrombus, and that we had adequately mobilized the IVC above the upper limit of the thrombus, the vessel loops were wrapped twice around the IVC above and below the thrombus and around the left renal vein. Despite angioembolization 1 week previously and lateral retraction of the kidney, the thrombus was found to extend far enough into the IVC to necessitate complete cross-clamping of the IVC; tangential exclusion of the thrombus alone, with the use of a laparoscopic Satinksy clamp, was not deemed feasible based on the results of intraoperative ultrasonography.
The vessel loops were used to create modified-Rummel tourniquets as described by Abaza [2], and the IVC was completely cross-clamped starting with the most cranial IVC vessel loop (Fig. 4). With the vessel loops cinched down completely and secured by Hem-o-Lok clips (Teleflex Inc., Limerick, PA, USA), a small incision was made in one of the gonadal vein stumps to test the hemostasis within the cross-clamped portion of the IVC. Because of continuous bleeding, the gonadal vein stump was religated, the tourniquets were released, and a careful search for another source of inflow to the IVC was initiated. Just above the left renal vein and below our cephalad tourniquet, a missed lumbar vein was identified entering into the IVC posteriorly. After this vein was ligated with clips and the IVC was reclamped, the second gonadal vein stump was incised, and no further venous flow was observed. As such, an incision was made in the wall of the IVC to expose the IVC thrombus, which was delivered intact on complete transection of the renal vein (Fig. 4).
After the IVC lumen was irrigated with heparinized saline, a Van Velthoven-style [3] double-arm 4-0 prolene suture was used to close the IVC in two layers, which maintained at least 50% luminal caliber (Fig. 4). Before the IVC was closed, the caudal IVC tourniquet was loosened to vent any clot or debris within the IVC and to test the closure.
After adequate hemostasis was achieved, the thrombus was transected at the level of the renal vein and placed into a small entrapment sac to prevent contamination of the peritoneal space. The right adrenal gland was taken en bloc with the kidney, which was then mobilized free of its remaining superolateral attachments. The specimen was removed through a modified-Gibson incision created by connecting the fourth robotic arm port and the 12-mm assistant port in the right lower quadrant.
The entire procedure was performed by two robotic surgeons early on in their learning experience (<25 robotic cases performed); console time was alternated between the two surgeons, and one surgeon remained at the patient's bedside at all times. The total operative time was 527 minutes, and the total IVC cross-clamp time was 15 minutes. Because of adhesions from a prior laparoscopic cholecystectomy and significant hepatomegaly, it took 90 minutes to mobilize the liver off the kidney, to place two laparoscopic locking graspers to retract the liver, and to manage a small liver laceration that occurred during mobilization; direct pressure with application of FloSeal (Baxter Healthcare Inc., Deerfield, IL, USA) was adequate for hemostasis.
The total estimated blood loss was 750 ml, half of which was accounted for by the IVC cross-clamp tests. Because the preoperative hemoglobin concentration was only 82 g/l, two units of packed red blood cells were transfused before the operation began, and two additional units were transfused postoperatively. The initial postoperative hemoglobin concentration was 90 g/l, and the hemoglobin concentration at the time of discharge was 95 g/l.
On completion of the procedure, a moderate-sized pneumothorax was identified on chest X-ray, which was likely related to the two locking graspers placed on the diaphragm and was used to retract the liver; therefore, a right-sided chest tube was required. The patient required 11.7 mg of a morphine-equivalent dose of intravenous narcotics during the initial 48 hours postoperatively and was then managed with oral antiinflammatory agents only. The patient was discharged home in stable condition on postoperative day 4.
The final histopathologic examination indicated clear cell RCC with rhabdoid differentiation (Fig. 4), evidence of tumor extension through the renal capsule into the perinephric fat, and thrombus positive for malignancy (pT3b). The surgical margins were negative, and the adrenal gland showed benign nodular hyperplasia.
The patient was found to have bilateral deep venous thrombus on postoperative day 11; thus, the patient was briefly admitted to the hospital for initiation of anticoagulation therapy. The patient was also referred to a hematologic oncologist for consideration of adjuvant therapy and is currently scheduled for restaging imaging at 8 weeks postoperatively.
Reports of the use of combined laparoscopic-open [4-6] and hand-assisted laparoscopic [7,8] approaches to treat renal tumors with an IVC thrombus have been reported. A single case report of a purely laparoscopic technique has also been published [9]. However, none of these cases required complete cross-clamping of the IVC. This technically challenging task has limited the application of MIS techniques to more advanced T3b tumors. The recently published case series of a purely robotic approach, however, did involve complete cross-clamping in 2 of 5 patients [2].
Although these reports indicate the successful use of MIS techniques to manage advanced renal tumors, critics continue to question the safety and general feasibility of such applications. Although it is true that the MIS approach to treat such complex tumors remains investigational, the advent of the robotic surgical platform may mitigate the technical challenges that prevent the acceptance and adoption of such approaches. Using an experienced laparoscopic bedside assistant and after significant preoperative preparation and planning, two relatively novice robotic surgeons were able to perform a purely robotic radical nephrectomy with caval thrombectomy requiring complete cross-clamping of the IVC, which was aided significantly by the improved precision and dexterity of the robotic platform.
Although the current case report and the case series reported by Abaza [2] provide initial validation of the feasibility of this technique, the utility of a purely robotic approach to T3b renal tumors undoubtedly remains highly investigational. The authors believe, however, that continued application of such innovative techniques is necessary to optimize the surgical care that we deliver to our patients.
Figures and Tables
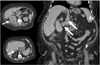 | FIG. 1Preoperative computed tomography images of a 12.0-cm right renal mass with inferior vena cava thrombus and a 3.5-cm right adrenal mass. |
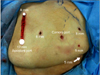 | FIG. 2Port placement for a robot-assisted laparoscopic radical nephrectomy with vena caval thrombectomy. |
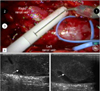 | FIG. 3Intraoperative ultrasonography was used to better characterize the inferior vena cava (IVC) thrombus. |
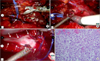 | FIG. 4Placement of modified-Rummel tourniquets around the inferior vena cava (IVC) (A), incision of the renal vein to deliver IVC thrombus (B), and closure of the IVC with the use of 4-0 prolene sutures (C). Histopathologic examination showed clear cell renal cell carcinoma with rhabdoid differentiation (D, H&E, ×10). |
References
1. Swierzewski DJ, Swierzewski MJ, Libertino JA. Radical nephrectomy in patients with renal cell carcinoma with venous, vena caval, and atrial extension. Am J Surg. 1994. 168:205–209.
2. Abaza R. Initial series of robotic radical nephrectomy with vena caval tumor thrombectomy. Eur Urol. 2011. 59:652–656.
3. Van Velthoven RF, Ahlering TE, Peltier A, Skarecky DW, Clayman RV. Technique for laparoscopic running urethrovesical anastomosis: the single knot method. Urology. 2003. 61:699–702.
4. Hoang AN, Vaporcyian AA, Matin SF. Laparoscopy-assisted radical nephrectomy with inferior vena caval thrombectomy for level II to III tumor thrombus: a single-institution experience and review of the literature. J Endourol. 2010. 24:1005–1012.
5. Varkarakis IM, Bhayani SB, Allaf ME, Inagaki T, Gonzalgo ML, Jarrett TW. Laparoscopic-assisted nephrectomy with inferior vena cava tumor thrombectomy: preliminary results. Urology. 2004. 64:925–929.
6. Disanto V, Pansadoro V, Portoghese F, Scalese GA, Romano M. Retroperitoneal laparoscopic radical nephrectomy for renal cell carcinoma with infrahepatic vena caval thrombus. Eur Urol. 2005. 47:352–356.
7. Sundaram CP, Rehman J, Landman J, Oh J. Hand assisted laparoscopic radical nephrectomy for renal cell carcinoma with inferior vena caval thrombus. J Urol. 2002. 168:176–179.
8. Martin GL, Castle EP, Martin AD, Desai PJ, Lallas CD, Ferrigni RG, et al. Outcomes of laparoscopic radical nephrectomy in the setting of vena caval and renal vein thrombus: seven-year experience. J Endourol. 2008. 22:1681–1685.
9. Romero FR, Muntener M, Bagga HS, Brito FA, Sulman A, Jarrett TW. Pure laparoscopic radical nephrectomy with level II vena caval thrombectomy. Urology. 2006. 68:1112–1114.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download