Abstract
Purpose
We evaluated the influence of urinary stone components on the outcomes of ureteroscopic removal of stones (URS) by electrohydraulic lithotripsy (EHL) in patients with distal ureteral stones.
Materials and Methods
Patients with a single distal ureteral stone with a stone size of 0.5 to 2.0 cm that was completely removed by use of EHL were included in the study. Operating time was defined as the time interval between ureteroscope insertion and complete removal of ureteral stones. Ureteral stones were classified into 5 categories on the basis of their main component (that accounting for 50% or more of the stone content) as follows: calcium oxalate monohydrate (COM), calcium oxalate dihydrate, carbonate apatite (CAP), uric acid (UA), and struvite (ST).
Results
A total of 193 patients (131 males and 62 females) underwent EHL. The mean operating time was 25.1±8.2 minutes and the mean stone size was 1.15±0.44 cm. Calcium oxalate stones accounted for 64.8% of all ureteral stones, followed by UA (19.7%), CAP (8.3%), and ST (7.2%) stones. The mean operating time was significantly longer in the UA group (28.6±8.3 minutes) than in the COM group (24.0±7.8 minutes, p=0.04). In multivariate analyses, the stone size was negatively associated with the odds ratio (OR) for successful fragmentation. UA as a main component (OR, 0.42; 95% confidence interval, 0.20 to 0.89; p=0.023) was also found to be significantly important as a negative predictive factor of successful fragmentation after adjustment for stone size.
Most urinary stones can be treated with extracorporeal shock wave lithotripsy (ESWL), but ureteroscopic removal of stones (URS) by use of intracorporeal lithotripsy devices has also been widely used as a valid, minimally invasive alternative to ESWL [1,2].
A gradual decrease in ureteroscope size and the development of effective lithotriptors are the most important technical advances in URS [3]. Smaller ureteral stones can be extracted intact with endoscopic baskets or grasping devices after ureteral dilation, if necessary. However, larger ureteral stones require lithotripsy for the safe extraction of the stone fragments. To date, four techniques are available for intracorporeal lithotripsy: electrohydraulic lithotripsy (EHL), laser lithotripsy, ultrasonic lithotripsy, and ballistic lithotripsy [4].
EHL was attempted in 1955 by Yutkin and was the first technique developed for intracorporeal lithotripsy (quoted from [5,6]). In recent years, the development of a new lithotriptor using a holmium: yttrium aluminum garnet (YAG) laser has increased the efficacy of URS for the treatment of ureteral stones. The holmium laser is one of the safest, most effective, and most versatile intracorporeal lithotripters. However, because of excellent flexibility and cost advantage, EHL has also been used for the treatment of ureteral stones [7]. The success rate of EHL is reported to be similar to that of laser lithotripsy [8]. It has been demonstrated that EHL successfully fragments 84 to 90% of stones without major complications [1,9,10]. To the best of our knowledge, there have been few reports regarding the correlation between stone components and the outcomes of URS using EHL in patients with distal ureteral stones, although a few previous studies have reported the outcomes of EHL.
The present study was conducted to evaluate the influence of ureteral stone components on the outcomes of URS by EHL in patients with distal ureteral stones.
We retrospectively analyzed the medical records of 193 patients (131 males and 62 females) who underwent URS by EHL for distal ureteral stones at our institution between March 2005 and April 2011.
Baseline demographic data and data on physical characteristics were obtained. The present study included patients with solitary, unilateral, distal ureteral stones with a stone size of 0.5 to 2.0 cm that were completely removed by URS by use of EHL. Patients who had tight stone impaction with significant tissue edema, inflammatory polyps, or ureteral strictures were excluded from the study. In addition, severe obesity, urinary tract malformations, ureteral strictures, active urinary tract infections, uncorrected coagulation disorders, previous stone manipulations, and previous ureteral surgeries were considered as exclusion criteria. The study protocol was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital. Stone size was defined as the largest diameter measured on abdominal computed tomography (CT) scans. The distal ureter was defined as the segment between the lower border of the sacroiliac joint and the ureterovesical junction in accordance with previous studies [2,11].
URS procedures were performed by a single urologist in the lithotomy position under spinal anesthesia. Access to the ureter was gained by using a 7.5-Fr rigid ureteroscope (Wolf, Knittlingen, Germany). The 7.5-Fr ureteroscope did not require dilatation of the ureterovesical junction. Guide wires were used selectively in some patients to facilitate access to ureteral stones. The EHL modality used was a Calcutript (Karl Stortz, Tüttlingen, Germany) electrohydraulic generator with a 3-Fr lithotriptor probe. All stones were fragmented under direct endoscopic vision, with simultaneous sterile saline irrigation using single pulses (frequency A) at level 2 intensity. Frasmented stones were extracted by using a 3-Fr zero tip nitinol stone retrieval basket (Boston Scientific, Natick, MA, USA). Operating time was defined as the time interval from ureteroscope insertion to the complete removal of stones.
Collected stones were analyzed according to a previous method [12]. Briefly, morphological examination of both the surface and sections of the stones was followed by sequential Fourier transform infrared (FT-IR) spectroscopic analysis, with quantification of stone components. Ureteral stones were classified into 5 categories on the basis of the main component accounting for 50% or more of the stone content as follows: calcium oxalate monohydrate (COM), calcium oxalate dihydrate (COD), carbonate apatite (CAP), uric acid (UA), and struvite (ST). We compared the outcomes of URS by EHL according to stone components.
The data are expressed as means±standard deviation. All statistical analyses were performed by using one-way analysis of variance, followed by the Bonferroni post hoc test for multiple comparisons and the Pearson chi-squared test for categorical variables. Univariate analysis was performed with Student's t- and chi-squared tests. Multivariate analysis was performed by means of logistic regression to assess predictive factors for successful fragmentation. SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) was used to perform all statistical analyses. Values of p<0.05 were considered statistically significant.
A total of 193 patients (131 males and 62 females) were studied. The mean age of the patients was 46.5±13.6 years. Patient demographics and stone characteristics are shown in Table 1. The mean operating time was 25.1±8.2 minutes (range, 10 to 55 minutes). The mean stone size as measured by abdominal CT was 1.15±0.44 cm (median, 1 cm; range, 0.5 to 2.0 cm). There were no major complications. Minor complications were hematuria (n=16) and postoperative ureteric colic (n=10), followed by fever (n=3), submucosal dissection (n=3), and minimal ureteric perforation (n=2). Double-J ureteric stents were placed as an auxiliary procedure in 14 patients (7.3%).
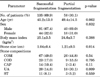
Calcium oxalate (CO) stones accounted for 64.8% of all ureteral stones. Of the CO stones, the proportions of COM and COD stones were 74.4% and 25.6%, respectively. The CO stones were followed by UA (19.7%), CAP (8.3%), and ST (7.2%) stones. The proportions of other chemical components are shown in Table 2.
Ureteral stones were classified into 5 categories on the basis of their main component (Table 3). There were no significant differences in sex or age between the individual groups (p=0.56 and p=0.79, respectively). The mean stone size was not significantly different between the individual groups (p=0.08). However, overall significant, between-group differences in the mean operating time were found (p=0.038). The mean operating time was significantly longer in the UA group (28.6±8.3 minutes) than in the COM group (24.0±7.8 minutes, p=0.04). No significant differences were observed between the other groups in terms of the mean operating time. Overall complication rates and the rates of each complication were not significantly different between the groups.
Cases with a mean operation time <30 minutes and residual fragments <3 mm were considered successful fragmentation, as previously described [13]. Univariate analysis of the factors showed that stone size and UA composition were associated with successful fragmentation by URS with EHL (Table 4).
In the multivariate analyses, the stone size was negatively associated with the odds ratio (OR) for successful fragmentation. UA as a stone component (OR, 0.42; 95% confidence interval, 0.20 to 0.89, p=0.023) was also significantly important as a negative predictive factor for successful fragmentation after adjustment for stone size (Table 5).
The purpose of this study was to evaluate the influence of ureteral stone components on the outcomes of URS by EHL in patients with distal ureteral stones. Ureteroscopic fragmentation of ureteral stones by use of intracorporeal lithotripsy is a popular, effective, minimally invasive technique and is considered to be the treatment of choice for lower ureteral stones [5]. Previous studies have reported success rates ranging from 72 to 100% [4,5,14,15], which is similar to the success rate in our study for patients who were treated by URS with EHL (98.5%).
EHL has been widely used for endoscopic treatment of urinary stones since its introduction by Yutkin in 1955 (quoted from [5,6]). Green and Lytton [16] reported their experience with EHL of ureteral stones with the use of a rigid ureteroscope and a 5-Fr EHL probe. Begun et al. [17] reported the use of a smaller EHL probe through a flexible ureteroscope. The initially developed EHL probe had a long diameter (9-Fr) and thus had a narrow margin of safety owing to its relatively large size. Later advances in technology have allowed for the development of 1.6- to 5-Fr probes, which are safer and are able to pass through short-diameter, flexible ureteroscopes. Fragmentation capability does not differ among probes, but larger probes appear to be more durable [14]. We used a 7.5-Fr rigid ureteroscope and a 3-Fr lithotriptor probe. Our surgical outcomes are in agreement with those of previous studies, regardless of probe size [15].
The efficacy of EHL in a stone is determined by the fragility of the stone and the pressure generated by the lithotripsy device [14]. We used a single pulse (frequency A) and a level 2 intensity in all cases. The collected stones were assessed by sequential FT-IR spectroscopic analysis and were categorized according to the main component. FT-IR spectroscopy is currently the most widely used method for investigating the composition and structure of urinary stones [18-20]. In the present study, most stones showed mixed components, and the most common component was CO, including COM and COD, which accounted for 64.8% of all ureteral stones. The proportions of stone components are consistent with those of previous studies [21,22].
In our analysis, stone components influenced the mean operating time, which was significantly longer in the UA group than in the CO group. Some studies have suggested that fragmentation failure may be due to a variety of stone components. Basar et al. [1] analyzed the fragmentation rate of 189 stones treated by EHL in relation to stone components. In that study, they reported that 7 cases were EHL-resistant and that fragmentation failure may depend on the proportions of CO and apatite and the organic matrix of the stones. Raney [23] performed electrohydraulic cystolithotripsy on 37 patients and suggested that urate, phosphate, and carbonate stones are easy to crush, whereas oxalate and UA are more resistant to fragmentation.
Other studies have demonstrated that fragmentation failure also depends on the surface characteristics of urinary stones. The more lamellated and harder a stone, the more difficult it is to break [1,4]. Stone surface characteristics may also play a role in fragmentation efficiency because rough stones have been reported to fragment more easily than smooth ones [1]. Stones with smooth and round surfaces are difficult to fragment because it is hard to keep a probe in contact with their surface. Although we did not analyze surgical outcomes according to stone surface characteristics, our results may be explained by the surface characteristics and properties of UA stones. UA stones usually form pebble-like objects with a smooth surface and are the hardest among human urinary stones [24]. A cross-section of the UA stone usually shows concentric layers of microcrystalline material that form typical laminations with radial striations converging to the core area [20,24]. This may also explain our results.
We found that the mean operating time increased with increasing percentage of UA in ureteral stones. UA stones exist most often in a pure state, and thus they are rarely accompanied by phosphate or calcium oxalate [24]. In our study, UA stones were more resistant to EHL fragmentation than were stones containing the other components.
Previous studies have reported that the major disadvantage of EHL is its propensity to damage the ureteral mucosa and its association with ureteral perforation and the risk of perforation, especially in impacted stones associated with significant mucosal edema [25]. We excluded impacted stones with tissue edema and inflammatory polyps from the study. Concerning postoperative complications, submucosal dissection occurred in 3 patients (1.6%) and minimal ureter perforation occurred in 2 patients (1.0%), but no major complications were observed. The reason for this lack of major complications and the lower prevalence of minor complications is that high-risk groups were excluded.
A limitation of our study is a lack of information about exact fragmentation time. Instead, we measured the mean operating time from ureteroscope insertion to the complete removal of ureteral stones. Considering ureteroscope manipulation time, the efficacy of EHL fragmentation may have been underestimated. However, most of the operating time as defined by our method was taken up by fragmentation; thus, the difference may be minimal.
To the best of our knowledge, there have been few reports on the influence of stone components on the outcomes of URS using EHL in patients with urinary stones. Despite the limitations of our study, our study has the strength of evaluating operating time in relation to stone components. Many recent studies have demonstrated that holmium: YAG laser lithotripsy is superior to EHL in terms of stone fragmentation and postoperative complications. This laser lithotripsy can also fragment hard UA stones that are resistant to EHL [3]. However, EHL is the cheapest intracorporeal lithotripsy method because it requires only an inexpensive generator and probes. For this reason, EHL is still widely used. EHL can fragment urinary stones, regardless of stone type, and can be a good alternative to laser lithotripsy. Stone components, especially UA, may influence the rate of successful fragmentation.
Figures and Tables
References
1. Basar H, Ohta N, Kageyama S, Suzuki K, Kawabe K. Treatment of ureteral and renal stones by electrohydraulic lithotripsy. Int Urol Nephrol. 1997. 29:275–280.
2. Verze P, Imbimbo C, Cancelmo G, Creta M, Palmieri A, Mangiapia F, et al. Extracorporeal shockwave lithotripsy vs ureteroscopy as first-line therapy for patients with single, distal ureteric stones: a prospective randomized study. BJU Int. 2010. 106:1748–1752.
3. Mugiya S, Nagata M, Un-No T, Takayama T, Suzuki K, Fujita K. Endoscopic management of impacted ureteral stones using a small caliber ureteroscope and a laser lithotriptor. J Urol. 2000. 164:329–331.
4. Defarges A, Dunn M. Use of electrohydraulic lithotripsy in 28 dogs with bladder and urethral calculi. J Vet Intern Med. 2008. 22:1267–1273.
5. See AC, Ng FC, Ch'ng HC. Electrohydraulic lithotripsy: an effective and economical modality of endoscopic ureteric lithotripsy. Aust N Z J Surg. 1997. 67:551–553.
6. Grocela JA, Dretler SP. Intracorporeal lithotripsy. Instrumentation and development. Urol Clin North Am. 1997. 24:13–23.
7. Mariani AJ. Combined electrohydraulic and holmium: YAG laser ureteroscopic nephrolithotripsy of large (>2 cm) renal calculi. Indian J Urol. 2008. 24:521–525.
8. Huang S, Patel H, Bellman GC. Cost effectiveness of electrohydraulic lithotripsy v Candela pulsed-dye laser in management of the distal ureteral stone. J Endourol. 1998. 12:237–240.
9. Noor Buchholz NP. Intracorporeal lithotripters: selecting the optimum machine. BJU Int. 2002. 89:157–161.
10. Yang SS, Hong JS. Electrohydraulic lithotripsy of upper ureteral calculi with semirigid ureteroscope. J Endourol. 1996. 10:27–30.
11. Tugcu V, Gurbuz G, Aras B, Gurkan L, Otunctemur A, Tasci AI. Primary ureteroscopy for distal-ureteral stones compared with ureteroscopy after failed extracorporeal lithotripsy. J Endourol. 2006. 20:1025–1029.
12. Daudon M, Lacour B, Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res. 2006. 34:193–199.
13. Nishizawa K, Yamada H, Miyazaki Y, Kobori G, Higashi Y. Results of treatment of renal calculi with lower-pole fluoroscopically guided percutaneous nephrolithotomy. Int J Urol. 2008. 15:399–402.
14. Elashry OM, DiMeglio RB, Nakada SY, McDougall EM, Clayman RV. Intracorporeal electrohydraulic lithotripsy of ureteral and renal calculi using small caliber (1.9F) electrohydraulic lithotripsy probes. J Urol. 1996. 156:1581–1585.
15. Gettman MT, Segura JW. Management of ureteric stones: issues and controversies. BJU Int. 2005. 95:Suppl 2. 85–93.
16. Green DF, Lytton B. Early experience with direct vision electrohydraulic lithotripsy of ureteral calculi. J Urol. 1985. 133:767–770.
17. Begun FP, Jacobs SC, Lawson RK. Use of a prototype 3F electrohydraulic electrode with ureteroscopy for treatment of ureteral calculous disease. J Urol. 1988. 139:1188–1191.
18. Hsu TH, Lin SY, Lin CC, Cheng WT. Preliminary feasibility study of FTIR microscopic mapping system for the rapid detection of the composited components of prostatic calculi. Urol Res. 2011. 39:165–170.
19. Daudon M, Jungers P. Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. Nephron Physiol. 2004. 98:p31–p36.
20. Fazil Marickar YM, Varma L, Koshy P. Ultrastructural study of laminated urinary stone. Urol Res. 2009. 37:289–292.
21. Wirtz P, Krambeck AE, Handa SE, Terry C, Lingeman JE. Contralateral ureteroscopy performed at percutaneous nephrolithotomy: a unique evaluation of stone-free rates. J Urol. 2010. 184:2378–2382.
22. Seitz C, Tanovic E, Kikic Z, Fajkovic H. Impact of stone size, location, composition, impaction, and hydronephrosis on the efficacy of holmium:YAG-laser ureterolithotripsy. Eur Urol. 2007. 52:1751–1757.
23. Raney AM. Electrohydraulic cystolithotripsy. Urology. 1976. 7:379–381.
24. Grases F, Villacampa AI, Costa-Bauza A, Sohnel O. Uric acid calculi: types, etiology and mechanisms of formation. Clin Chim Acta. 2000. 302:89–104.
25. Hofbauer J, Hobarth K, Marberger M. Electrohydraulic versus pneumatic disintegration in the treatment of ureteral stones: a randomized, prospective trial. J Urol. 1995. 153(3 Pt 1):623–625.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download