Abstract
Purpose
To report our technique for and experience with robot-assisted laparoscopic radical cystectomy (RARC) with orthotopic neobladder (ON) formation in a cohort of bladder cancer patients.
Materials and Methods
Between December 2007 and December 2011, a total of 35 patients underwent RARC. The patients' mean age was 63.3 years and their mean body mass index was 23.7 kg/m2. Thirty patients had a clinical stage of T2 or higher. Postoperative mean follow-up duration was 25.5 months. In 5 patients, a 4-cm midline infraumbilical skin incision was made for an ileal conduit (IC) and the stoma formation was similar to the open procedure. In 30 patients undergoing the ON procedure, the skin for specimen removal and extracorporeal enterocystoplasty was incised infraumbilically in the early 5 cases with redocking (ON-I) and suprapubically in the latter 25 cases without redocking (ON-S).
Results
The mean operative times of the IC, ON-I, and ON-S groups were 442.5, 646.0, and 531.3 minutes, respectively (p=0.001). Mean console and lymph node dissection time were not significantly different between the groups. Mean urinary diversion times in each group were 68.8, 125.0, and 118.8 minutes, respectively (p=0.001). In the comparison between the ON-I and ON-S group, only operative time was significant. Four patients required a blood transfusion. We had no cases of intraabdominal organ injury or open conversion. Thiry-three patients (94.2%) had a pathologic stage of T2 or higher. Two patients (5.7%) had lymph node-positive disease. Postoperative complications included ileus (n=4), stricture in the uretero-ileal junction (n=2), and vesicovaginal fistula (n=1).
Radical cystectomy is the gold standard for the treatment of muscle-invasive bladder cancer and noninvasive bladder cancer refractory to intravesical therapy [1,2]. Over the past decade, surgical techniques for open radical cystectomy (ORC) have improved dramatically, resulting in significant reductions in intraoperative blood loss and surgical morbidity. Despite these improvements, radical cystectomy remains a high-risk procedure with complication rates ranging from 30 to over 50% [3,4].
Laparoscopic surgery for the treatment of renal and renal pelvis tumors has been extensively applied without compromising the opportunity for cure. However, laparoscopic pelvic surgery has been limited to highly skilled laparoscopic surgeons. Although the feasibility of laparoscopic radical cystectomy (LRC) has been demonstrated [5-7], the benefit of this procedure has yet to be established. Owing to the technical challenges of the long operative time and difficulty of instrument handling, LRC is not yet in widespread use.
Currently, more than 60% of all radical prostatectomies in the United States are performed robotically [8]. If this trend continues, it is likely that robotic cystectomy will also grow in popularity. Recent reports have suggested that robot-assisted laparoscopic radical cystectomy (RARC) appears to be favorable with acceptable operative, pathologic, and short-term clinical outcomes [9-12]. The aim of the present study was to present our experience with and surgical technique for RARC.
Between December 2007 and December 2011, a total of 35 patients underwent RARC at our institution with the da Vinci robot system (Intuitive Surgical, Sunnyvale, CA, USA). All operations were performed with four robotic arms by two surgeons who had extensive operative experience. The following parameters were recorded and analyzed retrospectively: patient's sex, diagnostic age, previous history of surgery, operative procedure, and clinical and pathological cancer stage. The 2002 tumor-node-metastasis classification system was used for staging. We excluded patients with a history of pelvic radiation treatment or who could not be placed in a steep Trendelenburg position. All patients were offered RARC and underwent preoperative staging work-up including laboratory tests, chest X-ray, abdominal and pelvic computed tomography, and transurethral resection of the bladder tumor.
The preoperative characteristics of the patients are listed in Table 1. The patients' mean age was 63.3 years (range, 40 to 81 years) and their mean body mass index was 23.7 kg/m2 (range, 17.3 to 29.6 kg/m2). The study group comprised 22 men and 13 women. The mean American Society of Anesthesiologists (ASA) score was 2.0 (range, 1 to 3). The ASA score has been shown to be a significant predictor of morbidity in surgical patients [13]. Our patients underwent RARC for the following clinical stages: 25 (71.4%) and 5 patients (14.3%) had T2 and ≥T3 disease, respectively. Five patients (14.3%) had carcinoma in situ and high-grade T1 disease. The mean postoperative follow-up duration was 25.5 months (range, 4.1 to 63.6 months).
All patients completed a mechanical bowel preparation with antibiotic coverage over 1 or 2 days before the operation. Antegrade rinses of the bowel and neomycin-erythromycin intestinal preparations were avoided. Recent studies have found no significant difference in the rate of postoperative wound infection, clinical anastomotic leaks, or intraabdominal abscesses between patients with and without antegrade bowel rinses [14,15]. Such preparations can also increase the risk of fluid imbalances.
On the morning of the operation, a broad spectrum antibiotic drug was administered intravenously. To prevent deep vein thrombosis, all patients had on bilateral elastic stockings.
Under general anesthesia, the patients were placed in the Trendelenburg position and were padded to prevent neuromuscular injury. A nasogastric tube was inserted at the beginning of the operation. In the female patients, the abdomen and vagina were then prepped and draped in the standard fashion. On the operative field, an 18 Fr Foley catheter was introduced into the bladder.
A six-port approach was used. A 12-mm incision was made in the midline at 2 cm above the upper umbilical margin. A Veress needle was used to obtain pneumoperitoneum; alternatively, a Hasson approach was used. A 12-mm camera port was inserted. Two 8-mm ports for robotic arms (the first and second arm) were bilaterally established at a point 9 cm away from the umbilicus in the lateral direction. An additional 8-mm port for the third robotic arm was placed at a point 8 cm lateral from the right-sided robotic port. A 12-mm assistant port was placed at a point 8 cm lateral from the left-sided robotic port. The other 5-mm assistant port for suction and irrigation was placed between the camera port and the left robotic arm port. After completion of port insertion, the patient was placed in a steep Trendelenburg position. The robotic system was docked and radical cystectomy was performed first. Afterward, pelvic lymphadenectomies were performed.
Cystectomy began with the dissection of the ureters. The ureters were identified as they crossed over the iliac arteries and dove inferiorly into the pelvis (Fig. 1A). More distally, they lie just lateral to the seminal vesicles in men. Once identified and followed toward the bladder in patients of either gender, the ureters were taken about 2 from their vesical insertion and clipped with a 10-mm Hem-o-lok clip (Weck Closure Systems, Research Triangle Park, NC, USA) (Fig. 1B). After a posterior dissection, a frozen section of each distal ureter was sent for pathologic analysis. Delayed delivery of a frozen section resulted from creating a hydronephrosis of ureters, which may have the advantage of easier ureteroileal anastomosis.
A posterior approach through the cul-de-sac allows for easier mobilization of the bladder. In male patients, the dissection was made between the rectum and the bladder. In female patients, the dissection was made between the rectum and the vagina.
In men, the dissection began in the plane behind the seminal vesicles at the level of the Douglas pouch (Fig. 2). It may help to retract the sigmoid posterosuperiorly and the bladder superiorly. A 6- to 8-cm incision was made through the peritoneum and then the bladder was lifted vertically by the fourth robotic arm. This shows an excellent, clear operative field. Lateral to it, the vascular pedicles to the bladder come into view. The surgeon then retracted a seminal vesicle tip medially and superiorly and working laterally to this, took one and the other pedicle with a Hem-o-lok clip.
In women, the uterus was retracted anteriorly and the sigmoid posterosuperiorly as necessary, and a Hem-o-lok clip was used to transect the infundibulosuspensory ligaments. Taking these ligaments with their associated ovarian vasculature allowed anterior retraction of the adenexal structures and exposure of the peritoneum overlying the posterior vaginal fornix. This could be identified by a sponge stick in the vagina. The surgeon gently scored the parietal peritoneum by electrocautery and then focused anteriolaterally.
In men, the anterior dissection was accomplished via a parietal peritoneal inverted-U-shaped incision incorporating the urachus and bladder that connected with the peritoneostomy made for the posterior dissection. The bladder was then dropped, the space of Retzius defined, and the puboprostatic ligaments exposed. The endopelvic fasciae and puboprostatic ligaments were incised and a dorsal vein stitch was placed and tied. The dorsal vein was cut by using an electrocautery scissor and the urethra exposed. The catheter was removed. At this point, after leaving just enough of a stump and then tieing with Vicryl 1-0 and clipping with a 10-mm Hem-o-lok clip to prevent urine spillage, the urethra was cut with scissors. Typically, a distal margin was sent for frozen section. The posterior of the prostatic pedicles was sharply incised, with nerve-sparing possible as clinically indicated. Follow-up procedures were similar to robotic radical prostatectomy operations.
In women, the ovaries and uterus were usually removed depending on the tumor stage, the age of the patient, and the need for reproductive function. After dissection of the ureters was completed, the uterus was anteverted with the help of the fourth robotic arm. The infundibulo-pelvic suspensory ligaments along with the ovarian pedicle were identified and divided close to the uterus by using Hem-o-lok clips. The uterine artery pedicle could also be skeletonized and clipped after it was identified and isolated. Once adequate hemostasis was achieved, the fourth robotic arm was used to retract the freely mobile uterus and the surrounding adnexa. The dissection of the bladder was performed lateral to the umbilical ligaments, which helped to isolate and define the vascular pedicles. After the round ligament was transected, the superior vesical pedicle was clipped and divided by using Hem-o-lok clips. The bladder was retracted by using the fourth arm with gentle traction, and the vascular pedicle was placed. An Endo-GIA stapler with a vascular load was deployed, and after carefully identifying surrounding external iliac vessels as well as the rectum, the stapler was fired and the pedicle separated. Alternatively, the inferior vesical arteries were identified and clipped by using individual Hem-o-lok clips.
In women, the uterus was retracted proximally with the fourth robotic arm. The junction between the vagina and the bladder can be identified by manually manipulating a sponge stick in the vagina. This incision was then extended into the vagina and on both sides distally to the vagina as a strip en bloc with the posterior bladder. The excellent visualization aids in avoiding the peri-vaginal tissue, thus respecting oncologic safety and allowing preservation of sexual function.
The specimen including the bladder and uterus with ovaries was placed in the endo-catch bag and the edges of the vaginal wall were closed by using the "clam shell technique" with a running interlocking suture.
The lymph node dissection (LND) was performed after the bladder specimen was placed in an endo-catch bag and pushed away from the pelvis. This approach allows clear visualization of the anatomy and helps in performing a proper LND. LND was typically started along the external iliac vessels from the node of Cloquet up to the common iliac artery, if necessary up to the aortic bifurcation, its lateral border being the genitofemoral nerve. Adequate hemostasis was obtained by using bipolar current to cauterize small vessels and Hem-o-lok clips for the lymphatics. After the obturator nerves and vessels of the lymphatic tissue were identified from the obturator fossa, the triangle of Marcille and along the internal iliac vessels was removed.
When an orthotopic neobladder (ON) is made as a modified Hautmann pouch with a single chimney, it is necessary to tunnel gently between the two peritoneal openings with the fourth robotic arm to create a channel for the sutured left ureter in advance to allow it to sweep over in a smooth arc from the left to the right side. Otherwise, in double chimney fashion, it is not necessary to move the left ureter into the right side.
Both ureters are then sutured at the end with Vicryl 2-0 (Fig. 3A). This helps the surgeon to easily find them in the procedure of neobladder formation.
At this point, the cut urethra was sutured with 6 sutures of Vicryl 2-0 for the purpose of the open technique of urethra-reservoir anastomosis (Fig. 3B). This procedure helps to facilitate the anastomosis of the urethra-reservoir after orthotopic bladder substitute. The robotic systems are then undocked. Open procedures are prepared to accomplish orthotopic bladder substitute and anastomosis.
In patients undergoing an IC procedure, a 4-cm midline infraumbilical skin incision was made for specimen removal and extracorporeal enterocystoplasty. The stoma formation was similar to that of the open procedure. The ureteroileal anastomosis was performed over a 6 Fr ureteral diversion stent (Cook Urological Inc., Spencer, IN, USA) with 4-0 PDS sutures. A stoma was formed with the right-sided robotic port for the second arm.
In patients undergoing the ON procedure, the skin for specimen removal and extracorporeal enterocystoplasty was incised infraumbilically in the early five cases and suprapubically in all later cases. The ON was made as a modified Hautmann pouch with a single or a double chimney (Fig. 4). An infraumbilical ON (ON-I) was made by using about 70 cm distal ileum extracorporeally after a 4-cm vertical midline incision below the umbilicus. The robotic system was then redocked after closure of the vertical midline incision (Fig. 5). Afterward, the anastomosis between the urethra and the ON was performed intracorporeally. On the other hand, for the suprapubic ON (ON-S), the anastomosis was performed as an open technique via a suprapubic incision without robot redocking (Fig. 6).
The patients were divided into three groups according to which procedure they underwent. The IC group consisted of 5 patients. The ON-I and ON-S groups consisted of 5 and 25 patients, respectively (Table 2). Operative time (including redocking of the robot system) was defined as the time from the initial incision to final closure of skin. Mann-Whitney U tests were used to compare the perioperative data according to the surgical techniques (IC, ON-I, and ON-S). Statistical analyses were performed with SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered statistically significant.
The perioperative data of the 35 patients are given in Table 3. Mean operative times in the IC, ON-I, and ON-S groups were 442.5, 646.0, and 531.3 minutes, respectively (p=0.001). Mean console and LND time were not significantly different between the groups. Mean urinary diversion times in each group were 68.8, 125.0, and 118.8 minutes, respectively (p=0.001). However, estimated blood loss was not significantly different between the groups. Time to oral intake and ambulation were not significantly different between the groups. Postoperative urethral catheterization time in the ON-S group was shorter than in the ON-I group (p=0.030). In the comparison between the ON-I and the ON-S group, only the operative time was significant. Four patients required blood transfusion owing to perioperative bleeding. We had no cases of intraabdominal organ injury or open conversion.
The pathologic data are listed in Table 4. Thirty-three patients (94.2%) were pathologic stage T2 or greater. Two patients (5.7%) had node-positive disease. A positive resection margin was shown in one patient (2.9%).
Postoperative complications included mild ileus (n=3), severe ileus (n=1), stricture in the left ureteroileal junction (n=2), and vesico-vaginal fistula (n=1). Mild ileus was successfully managed with conservative treatment. The case of severe ileus required a surgical exploration by a general surgeon. The strictures in the ureteroileal junction and vesico-vaginal fistula required surgical revisions postoperatively. One patient with vesico-vaginal fistula repair surgery died. Thus, Clavien grade III and over complications remained in 4 cases (11.4%).
Radical cystectomy is the gold standard for treatment of muscle-invasive bladder cancer and noninvasive bladder cancer refractory to intravesical therapy [1,2]. Despite these improvements of surgical techniques, radical cystectomy remains a high-risk procedure with complication rates ranging from 30% to over 50% [3,4].
Minimally invasive surgery may reduce the morbidity associated with radical cystectomy, although this must be done without hampering the oncological outcome compared to open surgery. Laparoscopic surgery for the treatment of urologic tumors has been extensively applied without compromising the opportunity for cure. Potential advantages to performing the operation laparoscopically are smaller incision, decreased pain, shorter recovery time, and less blood loss, thus minimizing fluid imbalance and reducing hospital stay. However, laparoscopic pelvic surgery has been limited to highly skilled laparoscopic surgeons. Also, the benefit of LRC has yet to be established. Early groups have raised concerns that these procedures are technically demanding, take a long operative time, and require great laparoscopic skills [16].
Since the introduction of the robotic-assisted technique in our institution in 2007, this robotic technique has become the preferred procedure in our department. There has been a tremendous increase in the number of robot-assisted prostatectomies in South Korea during the past several years [17]. Thus, at our institution, surgeons have become skilled in the use of robotic surgery. If the trend for robotic surgery continues, it is likely that robotic cystectomy will also grow in popularity. Recent reports suggest that RARC appears to be favorable with acceptable operative, pathologic, and short-term clinical outcomes [9-12]. These studies confirm that robot-assisted cystectomy has the same potential advantages as conventional laparoscopic series compared to open surgery. However, RARC is still the most difficult procedure being performed in laparoscopic operative centers.
The first case report of RARC with intracorporeal ON was reported by Beecken et al. [18] in 2003. However, the intraabdominal formation of the ON is a very difficult procedure with robotic assistance. Subsequently, Hemal et al. [19,20] reported the technique of RARC with extracorporeal urinary diversion and intracorporeal neobladder-urethral anastomosis in men and women (sandwich technique). They demonstrated that this technique decreases operative time, morbidity, and complications. We also used this technique in our first 5 patients (ON-I). However, this sandwich technique requires a time of skin closure and redocking. Because of the reservoir tension in the patient's head direction, this technique was associated with difficulty in urethra-reservoir anastomosis and was a time-consuming procedure. To eliminate this time, we selected the alternative technique in later cases. Without redocking of the robotic system, the neobladder-urethral anastomosis was performed by the open technique (ON-S). The ON-S procedure significantly decreased the operative time (Table 3).
We performed 13 female cystectomies. Cystectomy in male and female patients differs with regard to the surgical approach. Female patients have a broader pelvis with more ready access to the apical/urethral dissection than do male patients. On the other hand, the female pelvic anatomy may be less familiar to urologic surgeons owing to the wealth of surgical experience in male patients, primarily because of the treatment of prostatic diseases and malignancies. Even with bladder cancer, the preponderance of patients is male by a ratio of 3 to 1 [21]. Furthermore, the female cystectomy procedure includes exenteration of the anterior pelvic organs, including the uterus, Fallopian tubes, ovaries, and occasionally part or all of the anterior vaginal wall. Such procedures can be associated with increased blood loss and added morbidity that has been observed in female patients versus male patients in ORC series by experienced surgeons [22]. Our initial experience with robotic-assisted laparoscopic anterior pelvic exenteration in female patients appears to be favorable, with acceptable operative, pathologic, and short-term clinical outcomes. As our experience increases, we should expect to continue to refine our surgical technique and reduce operative times. Certainly, a larger number of cases are required to adequately evaluate and validate this procedure as an appropriate surgical and oncological option for bladder cancer patients.
In our study, the urinary diversion time was not significantly different between the groups (p=0.089). However, this significance was shown in comparison of the ON-I and ON-S groups. A significant difference in total operation time was found between the ON-I and ON-S groups (p=0.048). This was because the neobladder-urethral anastomosis in the ON-I group was performed by the intracorporeal technique. Our experience shows that this procedure can be performed in well-described surgical steps that allow the operation to be accomplished in a reproducible and increasingly efficient manner, achieving and maintaining all of the principles and practices of the open principles of urinary diversion.
Catheterization time in the ON-S group was shorter than in the ON-I group (18.4 vs. 22.8 days). This was because a patient with vesico-vaginal fistula was included in the ON-I group. However, there was no significant difference between the ON-I and ON-S groups in catheterization time.
A majority of patients (85.7%) had pathologically invasive tumors (Table 4). Two patients with a result of pathologic N1 disease in the IC group received adjuvant chemotherapy, whereas no patients in the ON group were node-positive. Only 1 patient in the IC group had a positive resection margin, and there was inadvertent entry into the bladder.
Evaluating surgery-related complications by use of an instrument such as the Clavien classification can aid in assessing surgical techniques and designing clinical trials [23]. In the largest consecutive RARC series (100 cases), Pruthi et al. [24] reported major surgical complications (Clavien grade III or greater) in 8% of their patients. The results from an open RC series by Shabsigh et al. [25] showed major surgical complications (Clavien grade III or greater) in 13% of their patients (153 of 1142). The more favorable results from Pruthi et al. [24] could likely be explained because only 13% of their patients had non-organ-confined disease and only 5% received neoadjuvant chemotherapy.
In our results, major surgery-related complications (Clavien grade III or greater) occurred in 11.4% of patients. The Clavien grade III and over complications were severe ileus (n=1), strictures in the left ureteroileal junction (n=2), and vesico-vaginal fistula (n=1). The case of severe ileus required a surgical exploration by a general surgeon. The strictures in the ureteroileal junction and vesico-vaginal fistula needed surgical revisions postoperatively. One patient with vesico-vaginal fistula repair surgery died.
RARC with intracorporeal urinary diversion has been reported [18,26]. However, this procedure has a long operation time. To reduce the operative time, most centers prefer an extracorporeal urinary diversion [9,27]. Suturing seems to be a difficult technique with the robot. In particular, the neobladder-urethral anastomosis is very hard. This is the result of severe tension of the neobladder because of the patient's steep Trendelenburg position. However, our ON-S technique without redocking eliminates this difficulty and decreases the operative time. Because we need the skin incision for specimen removal, the use of it makes the operation easy. We propose that beginners perform the robotic neobladder with suprapubic incision by the open technique. This is especially true for surgeons without previous laparoscopic experience. To our knowledge, we have reported the first approach to RARC with extracorporeal neobladder formation and neobladder-urethral anastomosis by the open technique.
The present study had several limitations. Our results are in part limited by the retrospective analysis. The most important evaluation of all cancer operations is oncologic outcome. However, we could not measure outcomes such as local recurrence or distant metastasis during the relatively short-term follow-up. Also, evaluation of functional outcome is needed. A randomized prospective comparative study with ORC is needed to evaluate and validate this procedure. Finally, we did not distinguish the gender in the analyses. At a later time, we should prepare a new study addressing the above items.
In conclusion, RARC is rapidly growing in popularity. However, it has not yet attained widespread attention in uro-oncology owing to the complex surgical technique. Our technique for robot-assisted neobladder formation with a suprapubic incision without redocking is feasible and more rapid than robot-assisted neobladder formation with an infraumbilical incision and robotic redocking.
Figures and Tables
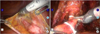 | FIG. 1Ureters dissection. (A) The right ureter was identified as it crossed over the external iliac artery. (B) The left ureter was dissected with cold scissors after clamping Weck clips. |
 | FIG. 3(A) Suturing of the ureter in order to easily find it during neobladder formation. (B) Suturing of the urethra to facilitate the urethra-reservoir anastomosis. |
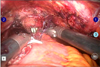 | FIG. 5Anastomosis of the urethra-reservoir in the fashion of the intracorporeal method after redocking the robot system. |
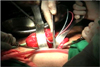 | FIG. 6Anastomosis of the urethra-reservoir in the fashion of the extracorporeal method by the open technique without redocking of the robot system. |
TABLE 2
Skin incision for specimen removal and extracorporeal enterocystoplasty in orthotopic neobladder (ON) patients

References
1. Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002. 167:1295–1298.
2. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001. 19:666–675.
3. Konety BR, Allareddy V, Herr H. Complications after radical cystectomy: analysis of population-based data. Urology. 2006. 68:58–64.
4. Novotny V, Hakenberg OW, Wiessner D, Heberling U, Litz RJ, Oehlschlaeger S, et al. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007. 51:397–401.
5. Finelli A, Gill IS, Desai MM, Moinzadeh A, Magi-Galluzzi C, Kaouk JH. Laparoscopic extended pelvic lymphadenectomy for bladder cancer: technique and initial outcomes. J Urol. 2004. 172(5 Pt 1):1809–1812.
6. Basillote JB, Abdelshehid C, Ahlering TE, Shanberg AM. Laparoscopic assisted radical cystectomy with ileal neobladder: a comparison with the open approach. J Urol. 2004. 172:489–493.
7. Haber GP, Colombo JR Jr, Aron M, Ukimura O, Gill IS. Laparoscopic radical cystectomy and urinary diversion: status in 2006. Eur Urol Suppl. 2006. 5:950–955.
8. Davis JW, Castle EP, Pruthi RS, Ornstein DK, Guru KA. Robot-assisted radical cystectomy: an expert panel review of the current status and future direction. Urol Oncol. 2010. 28:480–486.
9. Kasraeian A, Barret E, Cathelineau X, Rozet F, Galiano M, Sanchez-Salas R, et al. Robot-assisted laparoscopic cystoprostatectomy with extended pelvic lymphadenectomy, extracorporeal enterocystoplasty, and intracorporeal enterourethral anastomosis: initial Montsouris experience. J Endourol. 2010. 24:409–413.
10. Kwon SY, Kim BS, Kim TH, Yoo ES, Kwon TG. Initial experiences with robot-assisted laparoscopic radical cystectomy. Korean J Urol. 2010. 51:178–182.
11. Park SY, Cho KS, Ham WS, Choi HM, Hong SJ, Rha KH. Robot-assisted laparoscopic radical cystoprostatectomy with ileal conduit urinary diversion: initial experience in Korea. J Laparoendosc Adv Surg Tech A. 2008. 18:401–404.
12. Pruthi RS, Stefaniak H, Hubbard JS, Wallen EM. Robot-assisted laparoscopic anterior pelvic exenteration for bladder cancer in the female patient. J Endourol. 2008. 22:2397–2402.
13. Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005. 101:622–628.
14. Zmora O, Mahajna A, Bar-Zakai B, Rosin D, Hershko D, Shabtai M, et al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg. 2003. 237:363–367.
15. Brownson P, Jenkins SA, Nott D. Mechanical bowel preparation before colorectal surgery: results of a prospective randomized trial. Br J Surg. 1992. 79:461–462.
16. Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, Abol-Ein H, et al. Robot-assisted radical cystectomy and urinary diversion in female patients: technique with preservation of the uterus and vagina. J Am Coll Surg. 2004. 198:386–393.
17. Lee YS, Han WK, Oh YT, Choi YD, Yang SC, Rha KH. Robot-assisted laparoscopic radical prostatectomy: four cases. Yonsei Med J. 2007. 48:341–346.
18. Beecken WD, Wolfram M, Engl T, Bentas W, Probst M, Blaheta R, et al. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol. 2003. 44:337–339.
19. Hemal AK, Abol-Enein H, Tewari A, Shrivastava A, Shoma AM, Ghoneim MA, et al. Robotic radical cystectomy and urinary diversion in the management of bladder cancer. Urol Clin North Am. 2004. 31:719–729. viii
20. Hemal AK, Kolla SB, Wadhwa P. First case series of robotic radical cystoprostatectomy, bilateral pelvic lymphadenectomy and urinary diversion with da vinci-s system. J Robot Surg. 2008. 2:35–40.
21. Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007. 178:2314–2330.
22. Lee KL, Freiha F, Presti JC Jr, Gill HS. Gender differences in radical cystectomy: complications and blood loss. Urology. 2004. 63:1095–1099.
23. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. 240:205–213.
24. Pruthi RS, Nielsen ME, Nix J, Smith A, Schultz H, Wallen EM. Robotic radical cystectomy for bladder cancer: surgical and pathological outcomes in 100 consecutive cases. J Urol. 2010. 183:510–514.
25. Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009. 55:164–174.
26. Sala LG, Matsunaga GS, Corica FA, Ornstein DK. Robot-assisted laparoscopic radical cystoprostatectomy and totally intracorporeal ileal neobladder. J Endourol. 2006. 20:233–235.
27. Cathelineau X, Jaffe J. Laparoscopic radical cystectomy with urinary diversion: what is the optimal technique? Curr Opin Urol. 2007. 17:93–97.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download