Abstract
Purpose
Materials and Methods
Results
Conclusions
Figures and Tables
 | FIG. 1Ultrasound evaluation of patients with isolated urinary stress incontinence during the rest position, maximal pelvic floor contraction, and a Valsalva maneuver. |
 | FIG. 2Ultrasound evaluation of patients with urinary stress incontinence and genital prolapse stage I/II during the rest position, maximal pelvic floor contraction, and a Valsalva maneuver. |
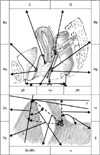 | FIG. 3Model of pelvic organ position during Valsalva maneuver vs. rest position in patients without urinary stress incontinence or genital prolapse. Dx, the distance between bladder neck (BN) and y-axis; Dy, the distance between BN and x-axis; Sy-BN, the distance between BN and inferior symphysis border; Distance-H (the height of BN), the distance between BN and horizontal line, which is drawn at the lower symphysis border; Distance R→V=(Vx-Rx)2+(Ry-Vy)2, angle of rotation-(ρ)=(cotangent Ry/Rx)-(cotangent Vy/Vx); Pubourethral angle-(α)- the distance between Sy-BN and x-axis, retrovesical angle-(β)- the angle for which one side lies along the line connecting the dorsocaudal and proximal urethra and the other side is formed by the tangent along the bladder base. |
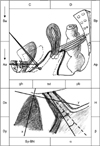 | FIG. 4Model of the possible movements of pelvic anatomical elements during Valsalva maneuver vs. rest position in patients with isolated urinary stress incontinence. Dx, the distance between bladder neck (BN) and y-axis; Dy, the distance between BN and x-axis; Sy-BN, the distance between BN and inferior symphysis border; Distance-H (the height of BN), the distance between BN and horizontal line, which is drawn at the lower symphysis border; Distance R→V=(Vx-Rx)2+(Ry-Vy)2, angle of rotation-(ρ)=(cotangent Ry/Rx)-(cotangent Vy/Vx); Pubourethral angle-(α)- the distance between Sy-BN and x-axis, retrovesical angle-(β)- the angle for which one side lies along the line connecting the dorsocaudal and proximal urethra and the other side is formed by the tangent along the bladder base. |
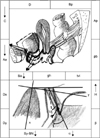 | FIG. 5Model of the possible movements of pelvic anatomic elements during Valsalva maneuver vs. rest position in patients with urinary stress incontinence and genital prolapse stage I/II. Dx, the distance between BN and y-axis; Dy- the distance between BN and x-axis; Sy-BN- the distance between bladder neck (BN) and inferior symphysis border; Distance-H (the height of BN), the distance between BN and horizonotal line, which is drawn at the lower symphysis border; Distance R→V=(Vx-Rx)2+(Ry-Vy)2, angle of rotation-(ρ)=(cotangent Ry/Rx)-(cotangent Vy/Vx); Pubourethral angle-(α)- the distance between Sy-BN and x-axis, retrovesical angle-(β)- the angle for which one side lies along the line connecting the dorsocaudal and proximal urethra and the other side is formed by the tangent along the bladder base. |
TABLE 2

POP-Q, International Continence Society's Pelvic Organ Prolapse Quantification system; USIGP group, USI/genital prolapse stage I/II; USI group, isolated urinary stress incontinence; t1, differences between column 1 and 2; t2, differences between column 3 and 4; t3, differences between column 1 and 3; t4, differences between column 2 and 4; Aa, a point located in the midline of the anterior vaginal wall 3 cm proximal to the external urethral meatus; Ba, the most distal position of any part of the upper anterior wall from the vaginal cuff to point Aa; C, leading edge of the cervix; D, the depth of the Douglas recession (distance between the hymen and the most distal point of the Douglas); Bp, the most distal position of any part of the upper posterior wall from the vaginal cuff to point Ap; Ap, a point located in the midline of the posterior vaginal wall 3 cm proximal to the hymen; gh, genital hiatus, measured from the middle of the external urethral meatus to the posterior midline hymen; tvl (total vaginal length), the greatest depth of the vagina in centimeters, i.e., the distance between hymen and point D; pb (perineal body), measured from the posterior margin of the genital hiatus to the midanal opening.
Student's paired test: a:p<0.05, b:p<0.001, c:p<0.01.
TABLE 3

USI group, isolated urinary stress incontinence; USIGP group, USI/genital prolapse stage I/II; RP, rest position; MPFC, maximal pelvic floor contraction; V, Valsalva maneuver; t4, differences between column 1 and 3; VM, Valsalva maneuver; t5, differences between column 4 and 6; t1, differences between column 1 and 4; t2, differences between column 2 and 5; t3, differences between column 3 and 6; Pubourethral angle-(α), the distance Sy-BN and x-axis; Retrovesical angle-(β), the angle which one side lies along the line connecting the dorsocaudal and proximal urethra, and the other side is formed by the tangent along the bladder base; Dx, the distance between blader neck (BN) and y-axis; Dy, the distance between BN and x-axis; H (distance H, i.e. the height of BN), the distance between BN and horisontal line, which is drown at the lower symphisis border; Sy-BN, the distance between BN and inferior symphisis border; Distance R→V (distance between Rest position and Valsalva maneuver), as a square root of the sum (Vx-Rx)2+(Ry-Vy)2, angle of rotation-(ρ)=(cotangent Ry/Rx)-(cotangent Vy/Vx).
Student's paired test: a:p<0.025; b:p<0.01; c:p<0.001; d:p<0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download