INTRODUCTION
Prostate cancer is a growing threat to Asian men's health [1]. Although the rate of incidence of prostate cancer is far greater in Western cultures (120 per 100,000 in Northern America) than in East Asian cultures (less than 10 per 100,000 in Asia) [2], when Asians migrate to Western countries, their rate of prostate cancer incidence increases. This finding supports the idea that lifestyle, diet, environmental, and genetic factors promote the development of prostate cancer [3,4]. This observation has prompted studies to identify dietary components, such as soy, a staple of the Asian diet, that may have anticancer properties. High soy intake may be one of the reasons Asians have a low risk of prostate cancer. Soy foods have been postulated to reduce the risk of several chronic diseases, including coronary artery disease, osteoporosis, and cancer. Moreover, the amount of soy taken in the diet is inversely proportional to the risk of prostate cancer [5-7]. The increasing burden of prostate cancer screening and treatment has made primary prevention an attractive strategy to decrease the incidence and slow the progression of this disease [8].
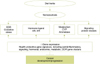
Chemoprevention is a prophylactic method that uses nontoxic natural or synthetic compounds to reverse, inhibit, or prevent the development of cancer by impeding specific molecular steps in the carcinogenic pathway. Prostate cancer has all the attributes of an ideal target disease for chemoprevention, including a long latency, high incidence, ample tumor marker availability, and identifiable preneoplastic lesions. Polyphenols, such as genistein, daidzein, equol, and curcumin (which are found in soy and the Indian spice, turmeric) are known to modulate multiple intracellular signaling pathways, including cellular proliferation, apoptosis, inflammation, and androgen receptor (AR) signaling, all of which have been implicated in cancer development and progression. These features make polyphenols promising chemopreventive agents (Fig. 1) [9,10].
Maintaining the integrity of the genome is fundamental to warding off cancer. Central to this process is the ability of cells to accurately recognize and correctly repair DNA damage in a timely manner to ensure the regulated and orderly progression of cells through the cell cycle. DNA damage caused by oxidative stress, chemical agents, or ultraviolet light triggers the DNA damage response (DDR). Recently, evidence has emerged that the DDR is one of the earliest events capable of thwarting the multistep progression of human epithelial carcinomas to invasive malignancy [11,12]. Agents that can activate the DDR are now appreciated as having potent cancer-preventive properties [13].
This review focuses on soy isoflavones and curcumin as candidate functional food factors that may be used for the chemoprevention of prostate cancer, especially emphasizing their effects on the DDR.
SOY ISOFLAVONES AND CURCUMIN: FUNCTIONAL FOOD FACTORS FOR CANCER PREVENTION
1. Soy isoflavones
In 2005, Yan and Spitznagel [14] published a meta-analysis of articles on the relationship between soy and prostate cancer. This analysis, which included 8 epidemiological studies, showed that the consumption of soy food was related to an approximately 30% reduction in prostate cancer risk. Additionally, soy consumption was correlated with a significantly lower mortality rate from prostate cancer. The protective properties of soy against prostate cancer are not only apparent in low-intake populations, but also among high soy consumers. Even among Japanese men with a relatively high soy intake compared with Caucasians, higher soy consumption is associated with a decrease in localized prostate cancer [15]. Studies in Asian men have reported that lower concentrations of equol, a metabolite of daidzein in the gut, or a lower prevalence of equol producers among men, also are risk factors for prostate cancer [16-19]. The ability to produce equol or equol, itself, may be closely related to the low incidence of prostate cancer in Asia [16,17]. Recently, it has been documented that the prevalence of equol production, a potential risk factor for prostate cancer, is decreasing in the young generations of Korea and Japan [20].
Soy isoflavones are classified as phytoestrogens. They have been suggested to modulate endogenous hormone homeostasis because their phenolic ring structures resemble estradiol and can bind to estrogen receptors, acting as either an estrogen agonist or an antagonist [21]. Soy isoflavones alter the activities of enzymes involved in hormone metabolism [22]. The primary isoflavones in soybeans are genistein, daidzein, and glycitein. Genistein, the predominant isoflavone in the human diet, is derived mainly from soybeans but is also found in other legumes, including peas, lentils, or other bean varieties [23].
Several mechanisms have been proposed to explain the anticarcinogenic activity of genistein: inhibition of protein-tyrosine kinase, with the result of alleviating the growth of cancer cells by inhibiting PTK-mediated signaling mechanisms; inhibition of topoisomerases I and II and protein histidine kinase, which have antiproliferative or proapoptotic effects; antioxidant effects, through inhibition of the expression of stress-response related genes; inhibition of nuclear factor kappa B (NF-κB) and Akt signaling pathways, both of which are important for cell survival; inhibition of angiogenesis; down-regulation of transforming growth factor-beta; and the inhibition of epidermal growth factor (EGF) [24].
2. Curcumin
Curcumin (diferuloylmethane) is a major chemical component of turmeric (Curcuma longa Linn.). Used as a spice in the Indian subcontinent, it adds flavor and yellow color to food. It has been used for centuries throughout Asia, not only as a food additive, but also in cosmetics and as a traditional herbal medicine, especially in the Ayurvedic, Chinese, and Hindu cultures, where it is used to treat a variety of inflammatory conditions and chronic diseases. Numerous studies have substantiated the anticarcinogenic properties and potential prophylactic or therapeutic value of curcumin [25]. Curcumin possesses antioxidant [26-28], anti-inflammatory [29], antiproliferative [30], and anti-angiogenic [31] properties. Curcumin, therefore, may also be an effective chemopreventive agent when used as a nontoxic dietary supplement [32].
MODE OF ACTION OF SOY ISOFLAVONES AND CURCUMIN ON PROSTATE CANCER
1. AR
Uncontrolled AR gene amplification, AR mutations, and increase of AR expression are a driving force in the progression of prostate cancer to the hormone-refractory state. Soy isoflavones, or genistein, down-regulate the AR and inhibit several steroid-metabolizing enzymes, such as 5-α-reductase or aromatase, thereby creating a more favorable hormonal milieu and a protective effect against prostate cancer. Soy isoflavones also block cell cycle progression at G1 and inhibit prostate-specific antigen (PSA) expression [23,33]. Curcumin down-regulates AR expression and AR-binding activity to the androgen response element of the PSA gene. It also down-regulates PSA expression in LNCaP cells [33-36]. Down-regulation of AR expression and blockage of its DNA-binding activity by curcumin lead to the inhibition of the homeobox gene NKX3.1 [37]. This gene plays a pivotal role in normal prostate organogenesis and carcinogenesis [38].
2. Cell proliferation and apoptosis
Soy isoflavones and curcumin inhibit the growth of LNCaP cells and DU 145 cells [39-41]. The combination of isoflavones and curcumin has a more potent inhibitory effect on cell proliferation than do isoflavones alone [41]. Curcumin induces apoptosis in prostate cancer cells in response to cellular signals including stress or DNA damage. Curcumin can down-regulate apoptosis suppressor proteins, such as Bcl-2 and Bcl-xL, up-regulate pro-apoptotic proteins from the Bcl-2 family (Bim, Bax, Bak, Puma, and Noxa), and activate caspases to induce apoptosis [34,42]. Curcumin also down-regulates murine double minute 2 (MDM2) protein and mRNA, an important negative regulator of the p53 tumor suppressor that permits prostate cancer cells to undergo apoptosis [43].
3. Protein kinases
Genistein inhibits activation of p38 MAPK and FAK (promotility proteins) in vivo and has been shown to block cell motility and prevent metastasis in an in vivo model [44]. The phosphatidylinositol 3-kinase (PI3 K)/Akt (protein kinase B)/mammalian target of rapamycin (mTOR) signaling pathway plays a central role in tumorigenesis and is often dysregulated in metastatic prostate cancers through the mutation of the phosphatase and tensin homolog, which leads to the constitutive activation of Akt [45]. Curcumin inhibits the phosphorylation of mTOR in prostate cancer cells [43,46] and PI3K [47]. Curcumin inhibits angiogenesis in vitro and in vivo [48,49]. The epithelial growth factor receptor (EGFR) family, including HER2, is an important mediator of cell proliferation and is highly expressed in prostate cancer cells, where it is associated with poor prognosis [50]. Curcumin is a potent inhibitor of EGFR signaling as it down-regulates EGFR expression and inhibits EGFR tyrosine kinase activity, as well as the ligand-induced activation of EGFR on LNCaP and PC-3 cells [51-54].
4. Cell cycle
Both soy isoflavones and curcumin have an impact on the cell cycle of prostate cancer cells. In one study, genistein caused dose- and time-dependent G2/M phase cell cycle arrest with increased WAF1/p21 and decreased cyclin B1 expression [47]. In another study, genistein reduced MDM2 protein and mRNA levels in prostate cancer cells by inducing ubiquitination of MDM2, which led to apoptosis, G2 arrest, and inhibition of cell proliferation [55]. Genistein has also been shown to up-regulate p21 in prostate cancer cells [56], whereas curcumin induces arrest in the G2/M phase in PC-3 and LNCaP cells [57].
Cyclin D1 is a proto-oncogene that plays an important role in cell proliferation through the activation of cyclin-dependant kinases and that is required for the progression of cells from the G1 to the S phase. Curcumin was shown to down-regulate cyclin D1 expression through activation of both transcriptional and posttranscriptional mechanisms in LNCaP prostate cancer cells [58]. A similar effect on cell cycle arrest in the G1/S phase was also observed in DU145 and PC-3 cells. This effect was observed not only through the up-regulation of the expression of cyclin-dependent kinase inhibitors p16, p21, and p27 and the inhibition of the expression of cyclin E and cyclin D1, but also through the hyperphosphorylation of retinoblastoma protein [59]. Curcumin inhibits prostate cell proliferation of LNCaP cell tumors implanted in nude mice, accompanying the induction of p21 and p27 and the inhibition of cyclin D1 [49,60]. These effects of soy isoflavones and curcumin lead to beneficial effects, including prostate cancer cell proliferation arrest and disruption of cell cycle control, resulting in cell death by apoptosis.
5. Inflammation
Inflammation is implicated as a major risk factor for prostate cancer. Cells that mediate inflammatory responses are major contributors to the in vivo production of reactive oxygen species, such as superoxide, nitric oxide, hydrogen peroxide, and hypochloric acid. High concentrations and the persistent presence of these reactive oxygen species are mutagenic and can lead to tissue damage. Population studies have found an increased relative risk of prostate cancer in men with prior histories of prostatitis (inflammation of the prostate) [61]. Inflammatory pathways including the cyclooxygenase-2 (COX-2) and NF-kB pathways are over-expressed in human prostate adenocarcinomas compared with normal prostate tissues [62,63]. The modulation of cellular signaling involved in the inflammatory response, especially targeting the NF-kB pathway, seems to be an important strategy for chemoprevention, because constitutive activation of NF-kB has been implicated in prostate cancer progression [64]. Genistein inhibits constitutive NF-kB/Ap-1 activities in prostate cancer PC3 cells [65]. These inhibitory effects are cancer-cell-specific because nontumorigenic prostate epithelial cells are not affected by genistein [65,66]. Finally, curcumin inhibits NF-kB activation in prostate cancer cell lines [42,67].
6. Antioxidant activity
Oxygen radicals may cause damage to DNA and chromosomes, induce epigenetic alterations, interact with oncogenes or tumor suppressor genes, and impart changes in immunological mechanisms that promote carcinogenesis. Genistein confers an antioxidant effect on prostate cancer cells by inducing glutathione peroxidase [68], whereas curcumin confers its antioxidant properties through the induction of phase II enzymes (glutathione S-transferase, heme oxygenase), especially by the regulation of the transcription factor Nrf2 (nuclear factor-erythroid 2-related factor 2 erythroid) [26,69].
CLINICAL APPLICATIONS
1. Soy isoflavones
Daily consumption of soy food decreases PSA levels in prostate cancer patients [70]. In a study in healthy Japanese volunteers, short-term administration of soy isoflavones stimulated the production of serum equol and decreased serum dihydrotestosterone levels [71]. In a phase II, randomized, double-blind, placebo-controlled trial, isoflavone (60 mg/day) or placebo was administered for 12 months to Japanese men between 50 and 75 years of age who had serum PSA levels between 2.5 and 10.0 ng/ml and a single negative prostate biopsy result within 12 months before enrollment. In that study, the incidence of cancer in the isoflavone group was significantly lower than in the placebo group for the patients aged 65 years or older [72].
2. Curcumin
Few clinical data about curcumin have been collected in humans, despite the large number of in vitro and animal studies. In a pilot study of a standardized oral Curcuma extract, doses of up to 180 mg of curcumin per day were administered to patients with advanced colorectal cancer for up to 4 months without overt toxicity or detectable systemic bioavailability [73]. A subsequent study suggested that doses of up to 8 g could be administered daily to patients with premalignant lesions for 3 months without overt toxicity [74]. The clinical advancement of this promising natural compound is hampered by its poor water solubility and short biological half-life, resulting in low bioavailability in both plasma and tissues [75]. In fact, after oral administration of free curcumin (up to 12 g/day), only nanomolar concentrations of curcumin or corresponding metabolites were found in patient serum [74,76]. On the other hand, low concentrations of curcumin were able to achieve a similar biological impact on NF-kB, COX-2, and phosphoSTAT-3 in peripheral blood mononuclear cells derived from treated patients compared with the ones observed in vitro with 550lM of curcumin [77,78].
3. Combined treatment with soy isoflavones and curcumin
In a double-blind, placebo-controlled study, subjects with high PSA levels but negative pathologic findings on prostate biopsy received either isoflavones (40 mg) and curcumin (100 mg) or placebo for 6 months. Isoflavones contained 66% daidzein, 24% glycitein, and 10% genistein. PSA levels were significantly decreased in the supplement-treated subgroup compared with the placebo group only in those subjects whose baseline PSA levels were over 10 ng/ml [33].
EFFECTS OF SOY ISOFLAVONES AND CURCUMIN ON THE DDR
Recently, evidence has emerged that the DDR is one of the earliest events that impedes the multistep progression of human epithelial carcinomas to invasive malignancy. DNA damage can be due to a variety of factors, such as telomere dysfunction or oncogene-induced replication stress [11,12]. The signaling pathways activated during the DDR have three critical goals: 1) halting cell cycle progression and division to prevent transfer of DNA damage to progeny cells; 2) increasing the accessibility of damage sites to (and engagement of) the DNA damage repair machinery, and 3) triggering apoptosis to exterminate cells whose damaged DNA cannot successfully be repaired [79].
The early precursor lesions commonly express markers of an activated DDR [11,12]. These markers include phosphorylated kinases (ataxia-telangiectasia-mutated kinase [ATM] and checkpoint kinase2 [Chk2]), phosphorylated histone H2AX, and phosphorylated p53. Such activation of the DDR network leads to senescence or death of oncogene-transformed cells that delays or prevents cancer progression. Epithelial cells within premalignant lesions with markers of senescence maintain an intact response to cellular stress and are less likely to develop subsequent tumors. However, the DDR is not only invoked but also dysfunctional at an early stage in the development of neoplasia. Markers of double-strand breaks, such as nuclear γH2AX foci (a histone phosphorylation event that occurs on chromatin surrounding a double-strand break), are markedly elevated in some precancerous lesions [80,81]. One hypothesis proposes that the original cause of these effects is oncogene activation [81]. The activation of oncogenes such as MYC and RAS stimulates the firing of multiple replication forks as part of a proliferative program. For precancerous lesions to progress to mature tumors, it is thought that critical double-strand break (DSB) signal transduction and cell cycle checkpoint proteins, such as ATM, ataxia telangiectasis Rad3-related (ATR), and the master "gatekeeper" protein p53, must become inactivated. With these DDR components rendered dysfunctional, collapsed forks are not effectively repaired, and cells proceed through the cell cycle with DNA lesions intact, thus increasing the chance of mutagenesis [80,81]. The 370 kDa Ser/Thr kinase ATM has begun to emerge as a central DNA damage checkpoint against cancer predisposition [82]. ATM is a member of the phosphoinositide 3-kinase-related protein kinase family to which the master regulator of cell growth and metabolism, the mTOR pathway, also belongs. ATM is a well-known primary regulator of the cellular response to DNA DSBs. Simultaneously, a number of studies have recently established that oxidative stress also activates ATM, even in the absence of DSBs. In response to oxidative stress, ATM is phosphorylated at Ser-1981, which results in phosphorylation of its substrates, including p53, the master controller of DNA metabolic stresses, and AMP-activated protein kinase-α (AMPKα), the key sensor of fuel and energy status [83].
Type 2 diabetes has been associated with elevated levels of DNA damage and with the decreased efficacy of DNA repair [83]. This can contribute to genomic instability in persons with type 2 diabetes and, consequently, to cancer promotion and progression. Indeed, cancer risk in type 2 diabetic patients is high. However, recent studies have shown that metformin significantly diminishes the risk of developing cancer in type 2 diabetic patients [84]. One causal linkage between metformin's mechanism of action and metformin's cancer preventative effects might be the activation of the DDR. Metformin promotes activation of ATM and ATM targets such as the protein kinase Chk2 [13].
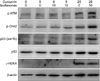
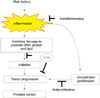
We recently reported that soy isoflavones and curcumin activate the DDR in LNCaP cells by inducing phosphorylation of ATM, histone H2AX, Chk2, and p53, which leads to apoptosis (Fig. 2). Combined treatment with isoflavones and curcumin conveys an additive effect. Interestingly, testosterone augments activation of the DDR as well as PARP cleavage induced by curcumin that is dependent on the AR. Thus, testosterone given in combination with polyphenol may have suppressive effects on the progression of prostate cancer [41]. Similarly, resveratrol, a polyphenol found in berries, nuts, and red wine, has been shown to activate the DDR pathways in ovarian carcinoma cells [85]. Because the DDR is the major innate tumor suppressor barrier in early human tumorigenesis, selective activation of DDR surveillance mechanisms by polyphenols may directly contribute to cancer-preventive effects (Fig. 3) [86].
CONCLUSIONS
Identifying the key proteins involved in carcinogenesis is an essential prerequisite to establishing an effective chemoprevention strategy. Soy isoflavones and curcumin can suppress not only the proliferation of both androgen-dependent and androgen-independent prostate cancer cell lines, but also that of xenografts by interfering with ARs, the cell cycle, activities of protein kinases, and apoptosis of prostate cancer cells. Furthermore, soy isoflavones and curcumin exert DDR effects in androgen-dependent prostate cancer cells. This provides a rationale for the clinical application of curcumin and isoflavones for the chemoprevention of prostate cancer.
To translate the in vitro efficacy of dietary agents in the prevention of cancer to clinical use, attention should be given to defining physiologically relevant concentrations and chronic exposure times under in vivo conditions. Indeed, it may not be practical to predict the chemopreventive effect of a single functional food factor. Rather, increased focus on the synergistic effect of treatment with combined functional food factors or multiple nutrients or food will yield a plausible approach to measuring the chemopreventive effect. On the basis of the epidemiologic and molecular evidence, the nutraceutical approach to cancer chemoprevention warrants further study.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download