Abstract
Hematogenous metastasis to skeletal muscle from urothelial carcinoma is extremely rare and metastatic disease to skeletal muscle tends to be found in people with advanced-stage neoplasm. We report in this paper a case of left sartorius muscle metastasis from urothelial cell carcinoma. A left nephroureterectomy with bladder cuff excision was performed and revealed a high-grade papillary transitional cell carcinoma (TCC) of the pelvis. And 6 month later, recurrent bladder cancer was found regular cystoscopy and then treated with transurethral resection of the bladder. After 6 times resection of bladder, an invasion into the bladder muscle layer was found. We recommended additional radical cystectomy to prevent the disease from advancing. However, the patient refused additional surgery. 6 month later, the patient complained of left thigh pain, so ultrasonography-guided biopsy of the nodular mass lesion in the left sartorius muscle was performed. The pathological analysis of the biopsy specimen revealed poorly differentiated metastatic urothelial carcinoma.
Hematogenous metastasis to skeletal muscle from urothelial carcinoma is extremely rare. We report a case of left sartorius muscle metastasis from urothelial carcinoma.
A 45 year old man presented with gross hematuria over the past 1 month. Ten years ago, he had undergone akidney transplantationowing to chronic renal failure. His family had a negative history of the disease. The physical examination revealed no specific findings except for the scar from the transplant operation. His serum creatinine on presentation was 1.7 mg/dl and urine analysis showed adequate red blood cells. There were no abnormal findings in the bladder during cystoscopy and he underwent an abdominopelvic computed tomography (CT) scan and retrograde pyelography, which revealed a 1.0×1.0 cm sized mass at the lower pole of the left renal pelvis (Fig. 1). The abdominopelvic CT scan, as performed, did not reveal metastasis to other organs or lymph nodes but there was a left renal pelvis mass. The bone scan did not reveal bone metastasis, and the chest X-ray did not reveal any particular findings. A left nephroureterectomy with bladder cuff excision was performed and the pathological analysis showed a high-grade papillary transitional cell carcinoma (TCC) of the pelvis. TNM staging was T2NOMO. Six months later, a regular follow-up cystoscopy confirmed four tiny papillary masses on the left lateral wall. Thus, he underwent transurethral resection of the bladder. Pathological tests showed a high-grade TCC, and the TMN staging was T1NOMO; Mytomycin-C instillation therapy was performed. The patient was under close follow-up. Clinical and radiological evaluations were done every 3 months in the first year. However, the bladder cancer recurred, and transurethral resection of the bladder tumor was performed six times in the next 5 years. The pathological analysis of the resected bladder mass revealed high-grade TCC. An invasion into the bladder muscle layer was found, and the TNM staging was T2N0M0. We recommended additional radical cystectomy to prevent the disease from advancing. However, the patient refused additional surgery because of his anxiety over losing a functional organ. He came in for dialysis owing to decreased renal function.
On the sixth month after the sixth transurethral resection of the bladder, a regular follow-up abdominopelvic CT revealed an enlarged lymph node near the right iliac vessel and multiple paraaortic and aortocaval lymphadenopathy with aggravated bladder wall thickening. In addition, the patient complained of left thigh pain; thus, magnetic resonance imaging (MRI) of the thigh was performed. The MRI showed a 3.1×6.5 cm sized oval-shaped T1 slightly high and T2 heterogenous high-signal intensity lesion in the mid portion of the sartorius muscle (Fig. 2). The lesion showed strong enhancement after contrast injection, which suggested intramuscular metastasis.
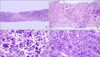
Ultrasonography-guided biopsy of the nodular mass lesion in the left sartorius muscle was performed (Fig. 3). The pathological analysis of the biopsy specimen revealed poorly differentiated metastatic urothelial carcinoma (Fig. 4). Chest CT and abdominopelvic CT showed lung, bone, and lymph node metastasis. Radiation therapy to the left metastatic skeletal muscle lesion and chemotherapy to the metastatic bladder cancer were performed.
Although skeletal muscle represents approximately 50% of the total body mass and receives a large portion of total cardiac output, hematogenous metastatic disease to the skeletal muscle is extremely rare. Several factors have been postulated to contribute to the resistance of skeletal muscle to metastatic disease. These factors include muscle motion and mechanical tumor destruction, inhospitable muscle pH, and the muscle's ability to remove tumor-produced lactic acid that induces tumor neovascularity in other tissues [1]. Despite these defensive factors, metastases to skeletal muscle have been reported from pancreatic, renal, colonic, pulmonary, gastric, and ovarian primary malignancies. The malignant tumor to muscle metastasis per se isevidence of systemic spread, and it is a worse prognosis than a metastasis to any other organ.
Metastatic disease to skeletal muscle tends to be found in people with advanced-stage neoplasms. The largest muscles, such as the erector spinae, psoas, and gluteals, are the most common sites of metastatic involvement.
The overall prevalence of skeletal muscle metastases in oncologic patients is not well reported. Subclinical metastases to skeletal muscle may indeed be more common than is generally thought. One large autopsy study of 5,298 people who died of malignancies found that 6% (298) involved the anterior chest wall or abdominal wall musculature and that 13% (666) involved the diaphragm [2]. Many of these lesions were microscopic and unlikely to be detected by any imaging technique.
In this case, the patient complained of muscular pain, which is compatible with studies showing that most lesions are painful. Skeletal muscle metastasis may be an incidental finding on CT of the chest or abdomen because most of the lesions identified were neither painful nor palpable.
The general CT findings of skeletal muscle metastasis show an increase in ring-shaped enhancement with central hypoattenuation, and MRI findings, although not specific, show low to intermediate signal intensity in the T1-weighted image and uniform high-signal intensity in the T2-weighted image. In this case, MRI showed a 3.1×6.5 cm sized oval-shaped T1 slightly high and T2 heterogenous high-signal intensity lesion in the sartorius muscle and strong enhancement after contrast injection, which suggested intramuscular metastasis. Biopsy can provide the appropriate diagnosis.
Methods for skeletal muscle metastases are still controversial. Ekici et al. [3] reported that if painful muscle metastasis is present, en bloc excision has good effects. Also, Pop et al reported that although not statistically significant, there was a slight difference in survival rates among patients who underwent radiotherapy or chemotherapy compared with those who underwent different surgeries [4]. However, Klune et al. [5] reported that surgical treatment was selectively possible only in patients meeting various conditions and recommended radiotherapy or chemotherapy.
Regarding bladder tumor metastasis to skeletal muscle, Nabi et al. [6] reported that the mean survival rate after receiving chemotherapy was 8 months, but because such cases are rare, specific details are not known. Di Giorgio et al. [7] reported that in the case of lung cancer metastasis to skeletal muscle, the survival rate is considerably reduced compared with the absence of metastasis, and in the case of three patients, the survival rate was not good at 3, 6, and 30 months.
Figures and Tables
FIG. 1
Retrograde pyelography (A) and abdominopelvic computed tomography (B) showing the mass at the left renal pelvis.
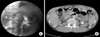
FIG. 2
Thigh MRI (A), whole-body bone scan (B), and PET-CT (C) showing a mass at the left sartorius muscle mass.
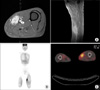
FIG. 3
Ultrasonography-guided biopsy of the left sartorius muscle consistent with metastatic urothelial carcinoma.

FIG. 4
The pathology of the left sartorius muscle biopsy specimen and bladder mass. (A) The needle-biopsied skeletal muscle showed metastatic tumor cells (H&E, ×40).Irregular clusters of poorly differentiated tumor cells infiltrated with desmoplastic are shown. (B) Reaction within the skeletal muscle fiber (H&E, ×100). (C) The tumor cells were polygonal or ovoid shaped and had abundant cytoplasm, irregular hyperchromatic nuclei, and occasional atypical mitoses (H&E, ×400). (D) Transurethral resection of the urinary bladder revealed infiltrative urothelial carcinoma, showing similar cytologic features with the tumor cells in the skeletal muscle (H&E, ×200).
References
1. Mulsow FW. Metastatic carcinoma of skeletal muscles. Arch Pathol. 1943. 35:112–114.
2. Pickren JW. Weiss L, editor. Use and limitations of autopsy data. Fundamental aspects of metastasis Amsterdam. 1976. Amsterdam: North-Holland;377–384.
3. Ekici S, Ozen H, Gedikoglu G, Aygün C. Skeletal Muscle Metastasis from Carcinoma of the Bladder. Scand J Urol Nephrol. 1999. 33:336–337.
4. Pop D, Nadeemy AS, Venissac N, Guiraudet P, Otto J, Poudenx M, et al. Skeletal Muscle Metastasis from non-small cell lung cancer. J thorac Oncol. 2009. 4:1236–1241.
5. Klune JR, Zuckerbraun B, Tsung A. Isolated skeletal muscle metastasis following successful treatment of laryngeal cancer: case report. Int Semin Surg Oncol. 2010. 7:1.
6. Nabi G, Gupta NP, Gandhi D. Skeletal muscle metastasis from transitional cell carcinoma of the urinary bladder: Clinicoradiological features. Clin Radiol. 2003. 58:883–885.
7. Di Giorgio A, Sammartino P, Cardini CL, Al Mansour M, Accarpio F, Sibio S, et al. Lung cancer and skeletal muscle metastase. Ann Thorac Surg. 2004. 78:709–711.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download