Abstract
Purpose
Primary culture of the cavernous smooth muscle cells from corpus cavernous tissues is known to be difficult, mainly because of contamination with fibroblasts. We applied a new method for better isolation of rat penile smooth muscle cells (RPSMCs) from rat corpus cavernosum tissue for reliable ex vivo research on erectile dysfunction.
Materials and Methods
With the use of 8-week-old adult male Sprague-Dawley rats, ex vivo migrations of rat cavernous tissue were measured by penis and aortic ring assay by use of a Matrigel-based D-valine-modified culture method. The expression of α-smooth muscle actin (α-SMA) and platelet/endothelial cell adhesion molecule (PECAM)-1 in the RPSMCs was determined by standard immunofluorescent staining and immunoblotting. The expression patterns of phosphodiesterase (PDE) family mRNA in RPSMCs were compared with patterns in rat aortic smooth muscle cells (RASMCs) by use of quantitative real-time reverse transcription polymerase chain reaction.
Results
Immunocytochemical staining showed greater α-SMA-positive and PCAM-1-negative fluorescence. Moreover, whereas the expression of α-SMA was detected in the RPSMCs, that of PECAM-1 was not. The levels of PDE1A, PDE1B, PDE1C, PDE2A, PDE3A, PDE4A, PDE4B, PDE4C, PDE4D, and PDE5A mRNA in the RPSMCs were about 3.2-, 4.4-, 3.4-, 29.0-, 3.5-, 2.8-, 2.9-, 6.1-, 45.0-, and 6.0-fold the corresponding expression in RASMCs.
The corpus cavernosum tissue consists of endothelial-lined sinusoidal structures surrounded by a specialized type of smooth muscle cells called corporal smooth muscle. Normal erectile function involves a coordinated relaxation of the cavernous arteries, cavernous sinusoidal endothelium, and the sinusoidal smooth muscle, which results in expansion of the cavernous sinusoids and an increase in intracavernous pressure. When penile erection is initiated, the cavernous smooth muscle cells relax and allow massive influx of blood into the sinusoidal spaces, which in turn causes rigid penile erection. The importance of smooth muscle relaxation in penile erection has been demonstrated in both animal and human studies [1,2]. The crucial role of the cavernous smooth muscle cells in penile erection is underscored by the fact that more than 60 to 80% of patients with erectile dysfunction (ED) can be treated by oral phosphodiesterase (PDE)-5 inhibitors that induce smooth muscle relaxation of the penis [3,4].
ED following radical prostatectomy (RP) results from inadvertent damage to the cavernous nerves that run close to the prostate capsule. The mechanisms behind the development of post-RP ED include cavernosal fibrosis and cavernosal smooth muscle apoptosis, resulting from cavernous nerve injury or neurapraxia during surgery [5,6]. In the hyperlipidemic state, lower numbers of neuronal nitric oxide synthase-positive nerves and a diminished endothelium can negatively affect erectile function because of the reduced bioavailability of nitric oxide. The diminished endothelium also causes the cavernous smooth muscle to lose contractility as the cells become synthetic [7,8]. Together, these structural changes may explain why men with cavernous nerve injury and hyperlipidemia are at higher risk of having ED.
Although animal models for ED have played a critical role in expanding our understanding of the pathophysiologic mechanisms involved in ED, it is necessary to establish a primary cavernous smooth muscle cell culture system from the rat corpus cavernosum tissue for the further delineation of the cellular and molecular mechanisms involved in smooth muscle cell dysfunction and ED. However, several technical difficulties remain in obtaining pure primary smooth muscle cells from corpus cavernosum tissue [9]. Impurity and dedifferentiation of isolated cells complicate the interpretation of results from cell culture-based experiments [10]. Matrigel is a gelatinous protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells. This mixture resembles the complex extracellular environment found in many tissues and is used by cell biologists as a substrate for cell culture [11,12]. Recently, Yin et al. [13] successfully isolated endothelial cells from mouse corpus cavernosum tissue by use of a Matrigel-based sprouting endothelial cell culture system.
In the present study, we developed a novel technique for the isolation of rat cavernous smooth muscle cells. We applied a two-stage tissue culture method with the use of a Matrigel-based sprouting culture system to facilitate stromal cell sprouting and an adherent culture system using D-valine to eliminate contamination of the smooth muscle cells with fibroblasts [14].
Eight-week-old adult male Sprague-Dawley rats (180 to 190 g, n=7) were purchased from Daehanbiolink (Chungju, Korea). Rats were housed under constant optimal temperature and humidity conditions with a normal 12-hour light-dark cycle. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996) and was approved by the Animal Subjects Committee of the Konkuk University School of Medicine, Korea. All experiments and animal care were performed in accordance with the institutional guidelines of Konkuk University. A mid portion of the corpus cavernosum tissue was harvested from each rat. Muscle strips from aortas were prepared in physiological salt solution (in mM; NaCl 136.9, KCl 5.4, CaCl2 1.5, MgCl2 1.0, NaHCO3 23.8, ethylenediaminetetraacetic acid 0.01), as reported previously [15,16].
Ex vivo migration of rat cavernous smooth muscle cells was measured by using Matrigel with the following modifications [13]. The corpus cavernosum tissue was harvested with the rats under anesthesia induced by intramuscular injections of ketamine (80 mg/kg) and xylazine (8 mg/kg). Briefly, the skin overlying the penis was incised and bilateral penile crura were exposed by removing part of the ischiocavernous muscle and fascia. After dissection and removal of the urethra, the corpus cavernosum tissues were obtained excluding the glans, cartilaginous portion of the penis, and the part of overlying ischiocavernous muscle. The corpus cavernosum tissue was washed in phosphate-buffered saline (PBS) several times. The samples (n=7) were enzymatically digested with collagenase (315 U/ml) and elastase (0.3 U/ml) for 25 minutes at 37℃ and were cut into fragments. The tissue fragments were then placed and embedded in 48-well plates coated with Matrigel supplemented with platelet-derived growth factor-BB and incubated for 5 days (Fig. 1). The sprouting cells were subcultured in Dulbecco's modified eagle medium (DMEM) containing 920 mg/l D-valine, 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 g/ml streptomycin, and 200 mM glutamine.
For all experiments, cultured rat penile smooth muscle cells (RPSMCs) were grown to 70 to 80% confluence and were starved in DMEM without FBS for 24 hours. After treatment with stimulants, cells were lysed with cold extraction buffer (20 mM HEPES, pH 7.5, 1% Nonidet P-40, 150 mM NaCl, 10% glycerol, 10 mM NaF, 1 mM Na3VO4, 2.5 mM 4-nitrophenylphosphate, 0.5 mM phenylmethylsulfonyl fluoride, and 1 tablet of complete proteinase inhibitor cocktail; Roche, Indianapolis, IN, USA). We also isolated rat aortic smooth muscle cells (RASMCs; passages 5 to10) from 6 rats and cultured the cells in DMEM containing 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 200 mM glutamine.
RPSMCs and RASMCs lysates were centrifuged (13,000×g, 15 minutes, 4℃), and the supernatants were collected as protein samples. Protein concentrations were determined by using Bio-Rad DC protein assay reagents. The protein homogenates were diluted 1:1 (v/v) with sodium dodecyl sulfate (SDS) sample buffer containing 40 mM Tris-HCl (pH 6.8), 8 mM ethylene glycol tetraacetic acid, 4% 2-mercaptoethanol, 40% glycerol, 0.01% bromophenol blue, and 4% SDS, and then boiled for 5 min. Proteins (30 to 50 g per lane) were separated by using 8 to 12% polyacrylamide SDS gels and were then transferred electrophoretically to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked for 2 hours at room temperature with PBS containing 0.05% Tween-20 and 5% fat-free dried milk. The membranes were incubated with primary antibody against α-smooth muscle actin (α-SMA) and platelet/endothelial cell adhesion molecule (PECAM)-1 (diluted 1:1000-2000) overnight at 4℃. Immune complexes were detected with horseradish peroxidase-conjugated antibodies (Amersham-Pharmacia, Piscataway, NJ, USA) diluted 1:1000 and incubated for 1 hour at room temperature. After application of the secondary antibody, blots were incubated in enhanced chemiluminescence kits (Amersham-Pharmacia) and exposed to LAS-3000. Band intensity was measured by computer analysis by using Quantitation software (Bio-Rad Laboratories Inc., Hercules, CA, USA).
The expression of α-SMA and PECAM-1 in the RPSMCs was determined by the standard immunostaining method. Briefly, the cells were fixed with 10% formaldehyde at room temperature for 15 minutes. After washing twice with PBS containing 0.05% Tween 20 and permeabilizing with 0.1% Triton X-100 for 5 minutes, the sprouts were treated with 1% bovine serum albumin in PBS for 2 hours and incubated with Alexa(594)-conjugated anti-α-SMA and Alexa(488)-conjugated anti-PECAM antibody (1:500; Sigma-Aldrich Co., St. Louis, MO, USA) overnight at 4℃. The immunostained sprouts were observed by use of fluorescence microscopy (Axio Observer A, Zeiss, Jena, Germany).
RNA was extracted by using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations. Total RNA (1 µg) was reverse-transcribed into cDNA by using the SuperScript III First-Strand Synthesis System for reverse transcription polymerase chain reaction (Invitrogen). Real-time RT-PCR was performed by using iQTMSYBR Green Supermix (Bio-Rad Laboratory Inc.) and iCycleriQ real-time PCR Detection System (Bio-Rad Laboratory Inc.). The relative mRNA expression level was determined by calculating the values of Δcycle threshold (ΔCt) by normalizing the average Ct value compared with its endogenous control (GAPDH) and then calculating 2-ΔΔCt [16]. All primer sequences are listed in Table 1.
Data are expressed as the mean±standard error. Statistical evaluation of the data was performed by using Student's t-tests for comparisons between pairs of groups and analysis of variance for multiple comparisons by use of GraphPad Prism version 4.0 (GraphPad Software Inc., La Jolla, CA, USA). p<0.05 was considered to indicate a significant difference.
To characterize primary cultured cells, we performed immunocytochemical staining for smooth muscle cell marker (α-SMA) and endothelial cell marker (PECAM-1). The majority of the cells showed positive staining for α-SMA but did not show positive fluorescence for PECAM-1 staining (Fig. 2). We also performed Western blot analysis in RPCMCs and RAMSCs to determine the protein expression of α-SMA and PECAM-1. Similar to the result from the immunocytochemical staining, both RPSMCs and RASMCs expressed α-SMA but did not express PECAM-1 (Fig. 3), which indicated that the primary cultured cells were smooth muscle cells.
To determine whether the expression patterns of PDE family mRNA differed between RASMCs and RPSMCs, we performed quantitative real-time PCR. The levels of calcium/calmodulin-dependent 3',5'-cyclic nucleotide PDE1A, PDE1B, and PDE1C; cGMP-dependent 3',5'-cyclic nucleotide PDE2A; cGMP-inhibited cyclic nucleotide PDE3A; cAMP-specific 3',5'-cyclic nucleotide PDE4A, PDE4B, PDE4C, and PDE4D; and cGMP-specific nucleotide PDE5A mRNA in the RPSMCs were greater by about 3.2-, 4.4-, 3.4-, 29.0-, 3.5-, 2.8-, 2.9-, 6.1-, 45.0-, and 6.0-fold, respectively, than in RASMCs (Fig. 4).
The development of various in vitro model systems from isolated corpus cavernosum tissues has tremendously enhanced our understanding of the biochemical and physiological mechanisms associated with erectile function. The main function of the corpus cavernosum tissues is to enhance blood flow into the penis through coordinated functions of the cavernous endothelial and smooth muscle cells, which are crucial for penile erection [17-19]. The corpus cavernosum tissue consists mainly of endothelial cells, smooth muscle cells, and fibroblasts. Whereas intact corporal tissue consists of a heterogeneous mixture of the cells, the isolation of pure primary cell components from erectile tissue may offer the possibility of defining the molecular and functional characteristics of particular cell types. However, previous studies aimed at the isolation of cavernous smooth muscle cells often lacked detailed isolation protocols or a precise characterization of the culture composition to exclude contamination from other cells, particularly fibroblasts [10]. The discrepancy in the purity of primary cultured smooth muscle cells in different reports might reflect laboratory and tissue source factors, a lack of precise exclusion of fibroblasts in other studies, and changes in properties of smooth muscle during the subcultivation. α-SMA, an isoform typical of SMCs and present in high amounts in vascular SMCs, was shown in the cytoplasm of pericytes of various rat and human organs by immunocytochemical staining. On the other hand, PECAM-1 is constitutively expressed on all vascular endothelial cells and has been regarded as a standard marker for endothelial cells in a variety of blood vessels, particularly in the setting of angiogenesis [20]. In the present study, the fluorescent immunocytochemistry of RPSMCs and RASMCs expressed α-SMA but did not express PECAM-1 (Figs. 2, 3). These results suggest that the isolated cells from penile tissues were not endothelial cells but were smooth muscle cells.
Research in tissue engineering, stem cells, or cell-based gene therapy primarily involves cultures of the cells on flat plastic dishes, and this technique is known as 2-dimensional cell culture. The majority of the current cell culture techniques are based on rigid and 2-dimensional substrates. Recently, the use of 3-dimensional cell culture systems has increased in research fields, including drug discovery, cancer biology, regenerative medicine, and basic life science research [12,13,21,22]. A variety of platforms are used to facilitate cell growth in a 3-dimensional cell culture system, such as nanoparticle-facilitated magnetic levitation, gel matrix scaffolds, and hanging drop plates. In this study, we applied unique two-stage cell culture methods by use of a Matrigel-based sprouting cell culture system and adherent cultures with D-valine. First, the corpus cavernosum tissue was implanted in Matrigel to facilitate stromal cell (smooth muscle cells and fibroblasts) sprouting. Second, the sprouting cells were subcultivated in DMEM containing 920 mg/l D-valine, 10% FBS, 100 U/ml penicillin, 100 g/ml streptomycin, and 200 mM glutamine. The cells were cultured in nutrient medium containing L-valine with D-valine added in an attempt to control fibroblast overgrowth. Contamination of RPSMCs cultures with fibroblasts was prevented in cultures maintained in D-valine but not in control cultures containing L-valine [14]. Our results showed that D-valine prevented fibroblast overgrowth without impairment of smooth muscle cell morphology or function.
The physiological importance of PDE5 in the regulation of smooth muscle tone has been demonstrated most clearly by clinical use of its specific inhibitors in the treatment of ED [23]. PDE5 inhibitor retards enzymatic hydrolysis of cGMP in the human corpus cavernosum, leading to dilation of the arteries that bring blood into the penis and inducing penile erection. Because PDEs form a biochemically and structurally diverse family of proteins, there may be more than one PDE isozyme or isogene that could serve as a potential drug target in the treatment of ED [24]. The PDE families include PDE1-Ca2+/calmodulin-dependent, PDE2-cGMP-stimulated, PDE3-cGMP-inhibited, PDE4-cAMP-specific, PDE5-cGMP-specific, PDE6-photoreceptor cGMP-specific, PDE7-cAMP-specific rolipram-insensitive, PDE8-cAMP-specific 3'isobutyl'1'methylxanthine-insensitive, PDE9-cGMP-specific, and PDE10-cAMP-inhibited family members. Different isogenes within a particular family are distinguishable by letters, for example PDE1A, PDE1B, and PDE1C [24]. Most mammalian tissues express several members of PDE families and many express more than 1 subtype of an individual family [24-26]. The presence of mRNA specific for 10 different PDE isozymes and isoforms in RPSMCs and RASMCs was shown by RT-PCR. We detected RPSMC and RASMC expression of genes encoding for cAMP and cGMP-hydrolyzing PDEs, including isogenes of PDE1; PDE2A (which hydrolyzes cAMP and cGMP); PDE3A; isogenes of PDE4 (the specific cAMP-hydrolyzing PDEs); and PDE5A (the specific cGMP-hydrolyzing PDE). In smooth muscle, nitric oxide regulates vascular tone by inducing relaxation through cGMP [27]. A previous study revealed that PDE isofoms are abundantly expressed in smooth muscle cells [28]. It was reported that PDE3 and PDE4 are highly expressed in the major cardiovascular and arterial system, whereas the expression of PDE5 is low [29]. However, our study showed a higher presence of mRNA of 10 PDE isogenes in RPSMCs compared with RASMCs (Fig. 4). This disparity may result from the differences in the preparation of specimens: our study used relatively pure RPSMCs or RASMCs, whereas the previous study [24,30] used organs, not smooth muscle cells. The presence of a variety of PDE isozymes in the primary cultured RPSMCs further supports the reliability of our protocol for the isolation of cavernous smooth muscle cells.
In this study, we developed a novel method for the isolation of RPSMCs by using a Matrigel-based sprouting culture system and subcultivation in DMEM containing D-valine. The in vitro model of primary cultured cavernous smooth muscle cells for the study of erectile function will give us important insights into the physiologic mechanisms of penile erection.
Figures and Tables
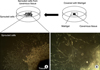 | FIG. 1Matrigel-based sprouting cavernous smooth muscle cell culture system in rats. The corpus cavernous tissue was implanted on a Matrigel-coated 60-mm cell culture dish with smooth muscle cell culture medium. After the cells were confluent and spread on the whole bottom, only sprouting cells were used for subculture. The photo was taken on day 5 after implantation. Magnification A, ×100; B, ×400. |
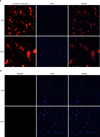 | FIG. 2Characterization of cultured rat penis smooth muscle cells (RPSMCs). The fluorescent immunocytochemistry of RPSMCs with anti-α-smooth muscle actin (A, positive marker) and anti-PECAM-1 (B, negative marker) is shown. Nuclei were labeled with the DNA dye DAPI. DAPI, 4,6-diamidino-2-phenylindole; PECAM-1, platelet/endothelial cell adhesion molecule-1. |
 | FIG. 3Western blotting analysis of rat penis smooth muscle cells (RPSMCs). Western blotting analysis was carried out to examine differences in α-smooth muscle actin and PECAM-1 in cultured RPSMCs and rat aortic smooth muscle cells (RASMCs). PECAM-1, platelet/endothelial cell adhesion molecule-1. |
 | FIG. 4Expression of different phosphodiesterase (PDE) isoforms in rat penis smooth muscle cells (RPSMCs) compared with rat aortic smooth muscle cells (RASMCs). The levels of PDE isoform mRNA in the SMCs were measured by real-time polymerase chain reaction: (A) PDE1A mRNA, (B) PDE1B mRNA, (C) PDE1C mRNA, (D) PDE2A mRNA, (E) PDE3A mRNA, (F) PDE4A mRNA, (G) PDE4B mRNA, (H) PDE4C mRNA, (I) PDE4D mRNA, (J) PDE5A mRNA. Each mRNA was normalized by that of GAPDH. a:p<0.05. |
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-521-E00089).
References
1. Martinez-Salamanca JI, Martinez-Ballesteros C, Portillo L, Gabancho S, Moncada I, Carballido J. Physiology of erection. Arch Esp Urol. 2010. 63:581–588.
2. Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989. 320:1025–1030.
3. Andersson KE. Neurophysiology/pharmacology of erection. Int J Impot Res. 2001. 13:Suppl 3. S8–S17.
4. Christ GJ, Hodges S. Molecular mechanisms of detrusor and corporal myocyte contraction: identifying targets for pharmacotherapy of bladder and erectile dysfunction. Br J Pharmacol. 2006. 147:Suppl 2. S41–S55.
5. Chung H, Lee CK, Kim B, Kim HS, Kim TW, Paick SH, et al. Proteomic analysis of penile protein alterations in a rat model of cavernous nerve injury. Korean J Urol. 2009. 50:498–504.
6. Koehler N, Holze S, Gansera L, Rebmann U, Roth S, Scholz HJ, et al. Erectile dysfunction after radical prostatectomy: the impact of nerve-sparing status and surgical approach. Int J Impot Res. 2012. 24:155–160.
7. Musicki B, Liu T, Lagoda GA, Strong TD, Sezen SF, Johnson JM, et al. Hypercholesterolemia-induced erectile dysfunction: endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J Sex Med. 2010. 7:3023–3032.
8. Qiu X, Fandel TM, Lin G, Huang YC, Dai YT, Lue TF, et al. Cavernous smooth muscle hyperplasia in a rat model of hyperlipidaemia-associated erectile dysfunction. BJU Int. 2011. 108:1866–1872.
9. Granchi S, Vannelli GB, Vignozzi L, Crescioli C, Ferruzzi P, Mancina R, et al. Expression and regulation of endothelin-1 and its receptors in human penile smooth muscle cells. Mol Hum Reprod. 2002. 8:1053–1064.
10. Pilatz A, Schultheiss D, Gabouev AI, Schlote N, Mertsching H, Jonas U, et al. Isolation of primary endothelial and stromal cell cultures of the corpus cavernosum penis for basic research and tissue engineering. Eur Urol. 2005. 47:710–718.
11. Benton G, George J, Kleinman HK, Arnaoutova IP. Advancing science and technology via 3D culture on basement membrane matrix. J Cell Physiol. 2009. 221:18–25.
12. Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010. 10:1886–1890.
13. Yin GN, Ryu JK, Kwon MH, Shin SH, Jin HR, Song KM, et al. Matrigel-based sprouting endothelial cell culture system from mouse corpus cavernosum is potentially useful for the study of endothelial and erectile dysfunction related to high-glucose exposure. J Sex Med. 2012. 9:1760–1772.
14. Hongpaisan J. Inhibition of proliferation of contaminating fibroblasts by D-valine in cultures of smooth muscle cells from human myometrium. Cell Biol Int. 2000. 24:1–7.
15. Lee CK, Han JS, Won KJ, Jung SH, Park HJ, Lee HM, et al. Diminished expression of dihydropteridine reductase is a potent biomarker for hypertensive vessels. Proteomics. 2009. 9:4851–4858.
16. Lee CK, Park HJ, So HH, Kim HJ, Lee KS, Choi WS, et al. Proteomic profiling and identification of cofilin responding to oxidative stress in vascular smooth muscle. Proteomics. 2006. 6:6455–6475.
17. Kimura M, Rabbani ZN, Zodda AR, Yan H, Jackson IL, Polascik TJ, et al. Role of oxidative stress in a rat model of radiation-induced erectile dysfunction. J Sex Med. 2012. 9:1535–1549.
18. Ozden E, Ozturk B, Kosan M, Tezel GG, Aki FT, Gur S, et al. Effect of sildenafil citrate on penile weight and physiology of cavernous smooth muscle in a post-radical prostatectomy model of erectile dysfunction in rats. Urology. 2011. 77:761.e1–761.e7.
19. Waldkirch ES, Uckert S, Sohn M, Kuczyk MA, Hedlund P. Rho kinase (ROK)-related proteins in human cavernous arteries: an immunohistochemical and functional approach. J Sex Med. 2012. 9:1337–1343.
20. Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Müller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002. 72:221–229.
21. Moreland RB, Hsieh G, Nakane M, Brioni JD. The biochemical and neurologic basis for the treatment of male erectile dysfunction. J Pharmacol Exp Ther. 2001. 296:225–234.
22. Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010. 5:291–296.
23. Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998. 159:2164–2171.
24. Kuthe A, Wiedenroth A, Magert HJ, Uckert S, Forssmann WG, Stief CG, et al. Expression of different phosphodiesterase genes in human cavernous smooth muscle. J Urol. 2001. 165:280–283.
25. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995. 75:725–748.
26. Manganiello VC, Murata T, Taira M, Belfrage P, Degerman E. Diversity in cyclic nucleotide phosphodiesterase isoenzyme families. Arch Biochem Biophys. 1995. 322:1–13.
27. Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000. 87:825–830.
28. Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003. 93:280–291.
29. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995. 75:725–748.
30. Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology. 2010. 59:367–374.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download