Abstract
Purpose
Chronic prostatitis (CP) does not yet have a universally successful therapy. Alternative treatments including thermotherapy have been adopted in the multimodal management of pain and voiding dysfunction. We retrospectively analyzed the therapeutic efficacy of bipolar radiofrequency thermotherapy for patients who were unsatisfied with conventional medication for CP.
Materials and Methods
A retrospective study between October 2009 and September 2010 of 26 patients who were under 50 years old and diagnosed with CP (National Institutes of Health [NIH]-category III) was performed. Twenty patients were diagnosed with inflammatory CP (NIH-category IIIa) and the rest with noninflammatory CP (NIH-category IIIb). We used the Tempro system at an intraprostatic temperature of 55℃ for 50 minutes with a medium heating rate. All patients also completed the NIH-Chronic Prostatitis Symptom Index (CPSI) before and after treatment.
Results
In the patients diagnosed with CP, the mean serum prostate-specific antigen (PSA) level was 0.9±0.3 ng/ml, the prostate volume was 27.1±5.5 g, and the average score for all 3 domains on the NIH-CPSI significantly decreased. The total scores decreased from 19.8±7.1 to 11.1±7.0, the pain domain decreased from 8.6±3.1 to 4.8±3.1, the voiding symptom domain decreased from 5.1±1.8 to 2.9±1.8, and the effect on the quality of life decreased from 6.1±2.2 to 3.4±2.2 (p<0.05).
As the third most common urological disorder in men over the age of 50 following benign prostatic hyperplasia (BPH) and prostate cancer, prostatitis represents 8% of all male urology office visits. It is also the most common urologic disorder in men under the age of 50 [1]. The accumulative lifetime chance of receiving a diagnosis of acute or chronic prostatitis by 85 years of age is 26% [2]. Category III prostatitis according to the National Institutes of Health (NIH) classification system is chronic nonbacterial prostatitis/chronic pelvic pain syndrome (CP/CPPS). Subgroups of this syndrome are categorized into category IIIa, or inflammatory CPPS, on the basis of the presence of excessive leukocytes in expressed prostatic secretions (EPS) or post-prostatic massage urine or semen and category IIIb, or non-inflammatory CPPS, which is defined by the lack of significant leukocytes in similar specimens.
Patients with CP/CPPS present with urological pain complaints as well as with voiding symptoms as a primary component of this syndrome. However, there is no universally successful therapy for CP/CPPS. Alternative therapeutic strategies and multimodal management may provide the best results at this time [3]. Among these therapies, heat therapy applied to the prostate gland may promote the biological process of fibrosis or scar formation in the chronic inflamed area and consequently could shorten the natural resolution of the inflammation [2]. We performed transurethral thermotherapy for patients who were diagnosed with CP and were dissatisfied with standard medication and analyzed the results to evaluate the therapeutic efficacy before and after treatment.
A retrospective study between October 2009 and September 2010 of 26 patients who had been diagnosed with CP was performed. The patients had been treated with conventional medical therapy such as α-adrenergic blockers, antibiotics, and/or anti-inflammatory agents for at least 3 months without improvement of symptoms. Twenty men were diagnosed with inflammatory CP (NIH-category IIIa) and the rest with non-inflammatory CP (NIH-category IIIb).
Initially, the serum prostate-specific antigen (PSA) level was tested to exclude prostate cancer. All patients also completed the NIH-Chronic Prostatitis Symptom Index (CPSI) before and after treatment. This index consists of the three domains of pain (location, frequency, and severity), voiding (irritative and obstructive symptoms), and quality of life, including impact. The NIH-CPSI has proven clinical importance in the evaluation and follow-up of patients in general urologic practice [4].
All patients discontinued conventional medication for CP at the beginning of the thermotherapy. We used the Tempro system from Direx-Initia at an intraprostatic temperature of 55℃ for 50 minutes with a medium heating rate and a single treatment session. This system includes the 6-ring electrode mounted on a silicone-coated 16 Fr latex Foley catheter and incorporates a computer-controlled radiofrequency (RF) generator that enables bipolar treatment. The treatment area is chosen according to the prostate urethral length as measured by transrectal ultrasound (TRUS) for localized treatment, which is effected by applying energy to different electrodes of the catheter. We also checked the prostate volume of the patients by TRUS before the treatment. Patient follow-up was done by 3 months so that patients could complete the NIH-CPSI after treatment. The baseline characteristics of the patients and their most bothersome symptoms can be seen in Table 1. For the subgroup analysis after treatment, we defined treatment success as a greater than 50% reduction in symptom scores.
For statistical analysis, we used the t-test, analysis of variance, and chi-square test for comparison of the differences in averages depending on times and groups. For all analyses, SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used, and p<0.05 was set for significance.
A total of 26 patients were diagnosed with CP and treated with bipolar RF thermotherapy as an in-office procedure conducted in a single session. Owing to the small diameter of the Foley catheter, its insertion is smooth and painless, and there is no give during the procedure. The patients' average age was 42.6±5.3 years (range, 32 to 50 years), and their mean serum PSA level was 0.9±0.3 ng/ml (range, 0.29 to 1.39 ng/ml). Mean prostate volume was 27.1±5.5 g (range, 17 to 38 g), and mean prostate urethral length was 3.6±0.3 cm (range, 2.8 to 4.2 cm). The most bothersome symptoms of the patients were pain and voiding symptoms (Table 1).
The average score for all 3 domains of the NIH-CPSI significantly decreased after treatment. The total scores decreased from 19.8±7.1 to 11.1±7.0, the pain domain decreased from 8.6±3.1 to 4.8±3.1, the voiding symptoms domain decreased from 5.1±1.8 to 2.9±1.8, and the effect on the quality of life decreased from 6.1±2.2 to 3.4±2.2 (p <0.05) (Fig. 1).
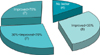
The average rate of improvement in the NIH-CPSI score after treatment was 43.9%: 44.2% in men with NIH-category IIIa and 42.9% in men with NIH-category IIIb; 7 of 26 men showed scores that markedly decreased by more than 70% after the thermotherapy (Fig. 2). When we defined the criterion of treatment success as a greater than 50% improvement in symptoms, as mentioned above, the number of patients in the more successful group (group A) and the number of patients in the less successful group (group B) was both 13. The baseline characteristics of the patients in group A and group B were not significantly different. Additionally, when we performed age- and prostate volume-specific analysis according to the significant difference in rate of improvement between the two groups, 9 patients were aged more than 40 years old with a prostate volume of less than 30 g, and 6 of these 9 men were assigned to group A. In other words, 67% of the 9 men were in group A, which is higher than the 41% of group A in the other categories, even if this was not statistically significant (p=0.465).
Acute complications after the treatment were 4 urinary tract infections treated with antibiotics and 1 "de novo" lower urinary tract symptom treated with conservative management (Table 2).
Prostatitis is a common urologic problem, and approximately 5% of visits to US urologists are reported to be for inflammatory diseases of the prostate [5]. Prostatitis also accounts for up to 15 to 25% of visits to urology clinics in South Korea [6]. Although it is suggested that prostatitis affects men of all ages, approximately 5% of young men between 20 and 50 years of age have suffered from prostatitis [7]. All men who participated in this study were less than 50 years old because of the differences in the prevalence of prostatitis and the symptomatic overlap between BPH and prostatitis over the age of 50 [1]. Nickel [8] found that approximately 5 to 20% of men diagnosed with BPH have prostatitis-like symptoms, with over one third of men with BPH also having prostatitis in the past. The disease is classified into four categories, including acute bacterial prostatitis, chronic bacterial prostatitis, CP/CPPS, and asymptomatic inflammatory prostatitis. The third disease category, CP/CPPS, accounts for approximately 90% of all chronic prostatitis cases and clinically presents with chronic pain in the perineum, rectum, penis, testicles, and abdomen [1]. It is often accompanied by symptoms of obstruction or irritative symptoms such as weak stream, hesitancy, and frequency on voiding. The symptoms usually remain stable or improve slightly over time, but some symptoms wax and wane over months in cycles [9].
CP/CPPS is considered to be a consecutive process caused by inflammatory, immunologic, neuroendocrine, and neuropathic mechanisms that begin with an initiator in a genetically or anatomically vulnerable man [10]. Although CP/CPPS is associated with bacteria in only about 8% of cases [11], infection has often been postulated to be an initiating factor [10]. Thus, medical therapy including antimicrobial or anti-inflammatory agents is started for patients diagnosed with CP/CPPS. However, the pain is observed to persist even after the absence of evidence of infection during follow-up. Urologists have difficulty in treating patients with CP/CPPS that is peripherally and centrally sensitized, and therapeutic mechanisms directed at the initiators of the process may not work as well when the condition becomes chronic [12]. Thus far, treatment strategies have focused on symptomatic relief.
Among the various strategic managements advocated for CP/CPPS, heat therapy in patients with poor response to standard therapies appears to be a promising therapeutic approach that has been reported to clinically reduce pain and voiding symptoms. Until now, whichever device was used, overall improvements with heat therapy have been reported in regard to both objective and subjective measures [13]. Heat therapy may modify the afferent nerve fibers that convey the objective symptoms of pain from the inflammatory processes of the prostate gland. Perachino et al. [14] proposed that transurethral thermotherapy induces a long-term alpha blockade. In addition, lower urinary tract symptoms have been consistently demonstrated to be associated with prostatic inflammation, and approximately 19% of men presenting with voiding symptoms as their chief complaint are diagnosed with prostatitis [15]. Brehmer and Baba [13] reported that urgency symptoms may be improved by decreased excitatory signals to the bladder with sensory denervation in the posterior urethra after the transurethral thermotherapy.
Prostate cells are not reliably destroyed until temperatures reach 45℃ [16]. Coagulative necrosis with cell death therefore begins at temperatures in excess of 45℃, with rapid thermoablation occurring at 60℃ or more [17]. The term thermotherapy was coined to describe treatment temperatures above 45℃ and the term hyperthermia is used for those below this level. Namely, the distinction between hyperthermia and thermotherapy is primarily their target temperature ranges, generally 42 to 44℃ for hyperthermia compared with temperatures of 45 to 50℃ for thermotherapy [18]. The treatment outcome of heat therapy for CP/CPPS shows inconsistent results according to the symptom measures used, temperature ranges, modes of treatment, and study design. The transrectal approaches report temperatures of 41 to 45℃, as do the transurethral microwave hyperthermia procedures.
Montorsi et al. [19] documented symptom improvement in 50% of men with chronic abacterial prostatitis by transrectal microwave hyperthermia at the long-term follow-up. Transurethral microwave thermotherapy (TUMT) studies have reported temperatures of 45 to 60℃, as have transurethral RF needle ablation (TUNA) procedures [18].
The clinical usefulness of TUMT for the treatment of symptomatic BPH has been reported in the urological literature, and TUMT has been widely used throughout the world. Because a RF electromagnetic wave has deeper and more even transmission of heat to tissues than does a microwave, transurethral RF thermotherapy for the treatment of BPH has shown comparable results to other nonsurgical treatment modalities [20]. The term radiofrequency refers not to the emitted wave but rather to the alternating electric current that oscillates in the high-frequency range (200 to 1,200 kHz). The RF energy flows through the electrodes and causes ionic agitation. This agitation and friction of ions creates heat and sufficient temperatures are reached.
The Tempro system is a transurethral heating procedure with temperatures of 45 to 60℃ for thermotherapy. The transurethral approach is considered to be a more direct way of heating than the transrectal approach. Bipolar technology enables concentrated RF transmission to the prostate with minimal energy delivery to the surrounding tissue. Furthermore, a cooling system is not required in this manner, whereas a microwave heating system, whether through a transrectal or transurethral approach, requires a cooling system because the lower tissue penetration requires greater power [21].
Nickel and Sorensen [22] examined the safety and efficacy of TUMT for nonbacterial prostatitis in 20 men randomly assigned to therapy or sham. At the 3-month follow-up, the patients treated by TUMT had significantly improved symptom scores compared with sham-treated patients. Seven of 10 men treated with TUMT had a favorable result compared with 1 of 10 men treated with a sham therapy. Aaltomaa and Ala-Opas [23] reported that TUNA relieved symptoms in CPPS patients and the need for medication was reduced. In this study, 27% of patients treated with transurethral RF thermotherapy showed marked improvement (symptom reduction of 70% or more), 27% showed moderate improvement (symptom reduction of 30 to 70%), and 31% showed mild improvement (symptom reduction 30% or less); 15% were no better. Whereas the mean rate of improvement in this study was 43.9%, Kastner et al. [24] reported the result of a pilot study in which 35 patients with CP showed improvement in the mean value of the NIH-CPSI of 51% with cooled TUMT through 12 months of follow-up.
In this study, patients with CP/CPPS were divided into two groups according to the rate of improvement after the treatment to analyze the factors that affect therapeutic efficacy, but we did not obtain significant results. In addition, considering that age and prostate volume can affect the rate of improvement, patients older than 40 years and with a prostate volume of less than 30 g showed a tendency for a better therapeutic effect, but the difference was not significant. Although we showed a therapeutic effect on symptom improvement, study of the factors affecting therapeutic results has been left for future evaluations.
Long-term study of the therapeutic effect of transurethral RF thermotherapy is also needed. Nickel et al. [25] studied the combined effects of RF thermal therapy and balloon dilation of the prostate. They evaluated 5 patients, and although there was some freedom from symptoms at 3 months, only 1 patient remained symptom-free for 6 months, and all patients were symptomatic at 9 months. Considering the characteristics of CP/CPPS, which has a chronic cyclic course, long-term follow-up will necessarily be needed. Among the multimodal approaches for patients suffering from this difficult condition, bipolar RF thermotherapy is a simple and effective treatment that can be tried as a second-line management following standard medication. Given that this study was conducted in patients who were unresponsive to conventional drugs, it is also fair to say that RF thermotherapy for CP/CPPS has therapeutic efficacy. Even though this was a short-term study with 3 months of follow-up, it showed significant improvement in pain and irritative voiding symptoms with a simple procedure and a minimal side effect profile, allowing that CP is a common male problem but is poorly managed. We suggest that transurethral RF thermal treatment could play a role in the management of refractory CP/CPPS, although the long-term effects of the therapy remain unclear.
CP/CPPS is a poorly defined disease entity with no definite treatment modalities. Of the multimodal strategies to improve pain and voiding symptom management for patients with CP/CPPS intractable to conventional medication, transurethral RF thermotherapy can provide significant improvement in the NIH-CPSI. It can also be an efficacious and simple alternative to treating CP/CPPS with minimal side-effects as an in-office procedure. Because long-term data documenting the durability of this improvement are currently unavailable, we need to conduct long-term follow-up of large-scale studies.
Figures and Tables
FIG. 1
Mean National Institutes of Health-Chronic Prostatitis Symptom Index (NIH-CPSI) scores before and after thermotherapy (p<0.05a). QoL, quality of life. a:t-test.

FIG. 2
Rate of improvement in National Institutes of Health-Chronic Prostatitis Symptom Index after thermotherapy (n=26).
References
1. Collins MM, Stafford RS, O'Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998. 159:1224–1228.
2. Nickel JC. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Inflammatory conditions of the male genitourinary tract: prostatitis and related conditions, orchitis, and epididymitis. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;304–329.
3. Shoskes DA, Hakim L, Ghoniem G, Jackson CL. Long-term results of multimodal therapy for chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2003. 169:1406–1410.
4. Nickel JC. Prostatitis: evolving management strategies. Urol Clin North Am. 1999. 26:737–751.
5. Schappert SM. National Ambulatory Medical Care Survey: 1991 summary. Vital Health Stat 13. 1994. (116):1–110.
6. Yoo YN. Prostatitis. Korean J Urol. 1994. 35:575–585.
7. Moon TD, Hagen L, Heisey DM. Urinary symptomatology in younger men. Urology. 1997. 50:700–703.
8. Nickel JC. The overlapping lower urinary tract symptoms of benign prostatic hyperplasia and prostatitis. Curr Opin Urol. 2006. 16:5–10.
9. Propert KJ, McNaughton-Collins M, Leiby BE, O'Leary MP, Kusek JW, Litwin MS, et al. A prospective study of symptoms and quality of life in men with chronic prostatitis/chronic pelvic pain syndrome: the National Institutes of Health Chronic Prostatitis Cohort study. J Urol. 2006. 175:619–623.
10. Pontari MA, Ruggieri MR. Mechanisms in prostatitis/chronic pelvic pain syndrome. J Urol. 2004. 172:839–845.
11. Nickel JC, Alexander RB, Schaeffer AJ, Landis JR, Knauss JS, Propert KJ, et al. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol. 2003. 170:818–822.
12. Yang CC, Lee JC, Kromm BG, Ciol MA, Berger RE. Pain sensitization in male chronic pelvic pain syndrome: why are symptoms so difficult to treat? J Urol. 2003. 170:823–826.
13. Brehmer M, Baba S. Transurethral microwave thermotherapy: how does it work? J Endourol. 2000. 14:611–615.
14. Perachino M, Bozzo W, Puppo P, Vitali A, Ardoino S, Ferro MA. Does transurethral thermotherapy induce a long-term alpha blockade? An immunohistochemical study. Eur Urol. 1993. 23:299–301.
15. Collins MM, Stafford RS, O'Leary MP, Barry MJ. Distinguishing chronic prostatitis and benign prostatic hyperplasia symptoms: results of a national survey of physician visits. Urology. 1999. 53:921–925.
16. Larson TR, Bostwick DG, Corica A. Temperature-correlated histopathologic changes following microwave thermoablation of obstructive tissue in patients with benign prostatic hyperplasia. Urology. 1996. 47:463–469.
17. Schulman CC, Zlotta AR, Rasor JS, Hourriez L, Noel JC, Edwards SD. Transurethral needle ablation (TUNA): safety, feasibility, and tolerance of a new office procedure for treatment of benign prostatic hyperplasia. Eur Urol. 1993. 24:415–423.
18. Zeitlin SI. Heat therapy in the treatment of prostatitis. Urology. 2002. 60:6 Suppl. 38–40.
19. Montorsi F, Guazzoni G, Bergamaschi F, Galli L, Consonni P, Matozzo V, et al. Is there a role for transrectal microwave hyperthermia of the prostate in the treatment of abacterial prostatitis and prostatodynia? Prostate. 1993. 22:139–146.
20. Terai A, Arai Y, Yamamoto I, Onishi H, Oishi K, Yoshida O. Newly developed transurethral radiofrequency thermotherapy device for benign prostatic hyperplasia: a pilot study in canine prostate. Int J Hyperthermia. 1995. 11:627–635.
21. Watson GM, Perlmutter AP, Shah TK, Barnes DG. Heat treatment for severe, symptomatic prostatic outflow obstruction. World J Urol. 1991. 9:7–11.
22. Nickel JC, Sorensen R. Transurethral microwave thermotherapy for nonbacterial prostatitis: a randomized double-blind sham controlled study using new prostatitis specific assessment questionnaires. J Urol. 1996. 155:1950–1954.
23. Aaltomaa S, Ala-Opas M. The effect of transurethral needle ablation on symptoms of chronic pelvic pain syndrome: a pilot study. Scand J Urol Nephrol. 2001. 35:127–131.
24. Kastner C, Hochreiter W, Huidobro C, Cabezas J, Miller P. Cooled transurethral microwave thermotherapy for intractable chronic prostatitis: results of a pilot study after 1 year. Urology. 2004. 64:1149–1154.
25. Nickel JC, Siemens DR, Johnston B. Transurethral radiofrequency hot balloon thermal therapy in chronic nonbacterial prostatitis. Tech Urol. 1998. 4:128–130.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download