Abstract
Purpose
To review our experience with the management of fragmented and retained pigtail percutaneous nephrostomy (PCN) tubes and to explore the reasons for the fragmentation.
Materials and Methods
We retrospectively reviewed our institute database from January 2006 to December 2011 for patients who had undergone retrieval of fragmented PCN tubes. We assessed the preoperative factors, operative technique, and post-operative outcomes.
Results
A total of seven patients (4 males and 3 females) had been diagnosed with fragmented PCN tubes. The mean age of the patients was 41.5 years. Of the seven patients, five required antegrade instrumentation by way of a percutaneous tract to remove the foreign body, mostly along with stone retrieval. One patient underwent ureterorenoscopy and pneumolithotripsy for a ureteric stone along with ureteroscopic removal of the PCN fragment. Another patient underwent nephrectomy of the kidney containing the PCN fragment because it had become nonfunctioning. All patients were free of stones and symptoms on follow-up.
Conclusions
A prolonged waiting period for definitive surgery, urinary infection, and associated stone disease are significant factors causing fragmentation of PCN tubes. Proper insertion techniques, regular timed changes of the PCN tube, appropriate care of the PCN tube, and early surgery for underlying stone disease are required to avoid this complication. Patients with retained PCN tubes can be managed effectively with antegrade or retrograde endoscopic techniques while definitive management of the primary pathology is carried out, without any additional morbidity.
Percutaneous nephrostomy (PCN) is a common procedure performed under fluoroscopic or ultrasonographic guidance for urinary diversion. PCN has a high technical success rate and a low complication rate [1]. Fragmentation of PCN tubes is a rare complication and is under reported in the literature. We present our experience of managing seven patients with broken PCN tubes lying in the pelvicalyceal system. To our knowledge, this is the first series documenting the management of retained fragmented nephrostomy tubes. We briefly explore the possible mechanisms of fragmentation and methods to prevent those.
We retrospectively reviewed our hospital database from January 2006 to December 2011 to identify those patients who had a fragmented PCN tube among those who had undergone PCN. The patient demographics, clinical presentation, baseline hematological and biochemical parameters, underlying primary pathology, urine culture reports, and possible etiology and mechanism of fragmentation were analyzed. The quality and the material of the tube used were analyzed. The radiological investigations used for diagnosis and the method of retrieval of the fragmented nephrostomy tube were noted. The follow-up details including radiological investigations and serum creatinine to assess renal function were noted.
Over 6 years, 1,220 patients had undergone PCN tube insertion our institute. Of these, seven patients had been diagnosed with fragmented pigtail nephrostomy tubes. There were four male and three female patients. The mean age at presentation was 41.5 years. Initial clinical presentation included ipsilateral flank pain in four, blockage in the nephrostomy tube in one, and fever with oliguria in another. Five patients had undergone PCN for renal stone disease with infected hydronephrosis, one patient for pyonephrosis, and the other for retroperitoneal fibrosis with persistent hydronephrosis and deranged renal function (Fig. 1). All the patients had been discharged home after the initial procedure of nephrostomy tube insertion. The time period that had elapsed between the insertion or change of the nephrostomy tube and presentation ranged from 21 to 86 days. In one patient, the PCN tube accidentally became fragmented and slipped inside the pelvicaliceal system during the initial placement by the interventional radiology team.
Initially, all patients were admitted and managed conservatively by antibiotics and subsequently underwent percutaneous retrieval of the fragmented tube during management of stone disease or nephrectomy. The broken fragments were retrieved successfully by a percutaneous technique under fluoroscopic guidance. The instruments used for the retrieval in the various patients were the standard 26 Fr rigid nephroscope, 15 Fr cystoscope, and 7.5 Fr ureteroscope (Fig. 2). Six patients were free of stones and fragmented nephrostomy tubes and one patient underwent nephrectomy for a non-functioning kidney associated with the fragmented nephrostomy tube. All patients were doing well after 6 months to 3 years of follow-up.
PCN is a simple, safe, and effective procedure for the management of patients with obstructive uropathy and is the treatment of choice in certain conditions like pregnancy and retroperitoneal fibrosis [1]. Complications are rare and usually occur at the time of tube insertion [2]. The associated mortality rate is approximately 0.04%, and the incidence of important complications is 5% [3]. Major complications of PCN include septicemia, hemorrhage, pneumothorax/hemothorax, and bowel injury [4,5]. PCN tubes are occasionally resistant to removal or become fragmented. The factors influencing tube fragmentation are the biomaterial used in manufacturing the tube, the technique of insertion, indwelling duration, concurrent metabolic abnormalities, and infection [3]. The incidence of fragmentation of polyurethane ureteral stents is 0.3% [6]. To our knowledge, no case series of patients with retained fragments of pigtail PCN tubes have been reported previously, although we believe that this does occasionally occur.
In all of our cases, the material used for the nephrostomy tube was polyurethane. Polyurethane is a polymer from a generic class of condensation polymers. Although it is highly versatile and inexpensive compared with other urinary tract biomaterials, polyurethane has been found to result in significantly more urothelial ulceration and erosion [7]. It is probable that cellular injury in response to the presence of urinary tract biomaterials may be an important determinant in the promotion and progression of encrustation, because many of these up-regulated proteins are also known for their role in wound healing [8]. Proper technique for renal access is essential to prevent complications [9]. We always used inferior calyceal puncture to access the pelvicaliceal system because it is associated with less chance of vascular and visceral injury. During the procedure, excessive torsion or bending in the tissue planes may lead to breakage of the tube. It has been suggested that fragmentation occurs at a site previously allowed to kink during stent insertion [10]. This could have been the reason for breakage in our case number four see Table 1.
Spontaneous fragmentation of three ureteral stents was reported by Zisman et al. [11]. The catheters were removed and were moderately encrusted. Electron microscopy of the cases reported by Zisman et al. revealed that all fractures passed across the side holes, which suggests that this area is a weak point conducive to kinking that may predispose to fragmentation [12]. This is relevant to PCN tube insertion also. In all of our patients, the breakage was either at the point where the coil starts from the straight tube or at the site of the side holes. Although bacteriuria is a major contributing factor, stent encrustation has also been observed with sterile urine cultures, indicating that there are additional causative factors.
An increase in the incidence of stent encrustation among chronic stone formers has been reported [12]. In our series, six out of seven patients had stone disease and four were recurrent stone formers. Associated urinary tract infections and persistent acidic urine have an impact on the strength and resiliency of the PCN tube. In our series, five out of seven cases had persistently positive urine cultures, commonly Escherichia coli and Proteus species.
Duration of indwelling is a very significant factor for breakage of PCN tubes. In this series, the duration ranged from 21 days to a maximum of 86 days. In a study of 290 patients with ureteral stents, el-Faqih reported that encrustation occurred in 9.2% of the polyurethane stents retrieved before 6 weeks, in 47.5% that were indwelling for 6 to 12 weeks, and in 76.3% thereafter [6]. We change the PCN tubes after 1 month, and the procedures are carried out in the intervention radiology and urology departments. The tubes are changed over a guide wire under fluoroscopic guidance.
In three of our cases we found the PCN fragment incidentally. The fragments had not been detected at the time of breakage and all three cases had been referred to us from other centers. In three of our cases we could detect the fragments immediately and appropriate measures were taken. Accordingly, when PCN tubes are changed, the pigtail coil should be inspected and the length should be measured to avoid this type of complication.
The techniques of retrieval in all of our cases differed. We used a 15 Fr cystoscope for removal of a PCN tube fragment and a small pelvi-ureteric junction stone. We used a 7.5 Fr semi-rigid ureteroscope for removal of another fragmented PCN tube associated with ureteric calculus. After ureterorenoscopy and pneumolithotripsy of the ureteric stone, the ureteroscope was able to reach the renal pelvis for retrieval of the PCN fragment. The nephroscope was used in cases in which it was associated with stones for intra-corporeal lithotripsy and stone retrieval along with PCN fragment removal. We used a 7.5 Fr ureteroscope in a case of retroperitoneal fibrosis in which we encountered a problem with dilation of the tract and we could dilate up to 16 Fr only.
This complication may be more common in developing countries owing to the delayed presentation with infection, lack of adequate follow-up, and long waiting periods in treating the underlying pathology, which is most frequently stone disease. Because our institute is in the region of a stone belt, we encounter a large burden of stone disease, especially that presenting late with obstruction and infection. This is reflected by the large volume of PCN tubes placed and the delay in treating the underlying disease. This may not be reflected in developed countries; nevertheless, it is important for urologists to be aware of this complication of a commonly performed procedure.
These cases highlight the need for careful inspection of the tip of the catheter and for careful noting of the details of the length of tubing at insertion and removal. A prolonged waiting period for definitive surgery, urinary infection, and metabolic diseases related to stone disease are significant factors in causing fragmentation of PCN tubes. Proper insertion techniques, regular timed changes of the PCN tube, appropriate care of the PCN tube, and early surgery for underlying stone disease are required to avoid this complication. This series also illustrates that this complication can be managed easily endoscopically while the primary pathology is tackled. Ongoing research in biomaterial science is essential to optimize biocompatibility and decrease biomaterial-related complications.
Figures and Tables
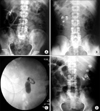 | FIG. 1(A) A fragmented and retained nephrostomy tube in the right kidney along with a fresh nephrostomy tube in situ in a patient with retroperitoneal fibrosis (case 1). (B) A patient with multiple right renal calculi and a fragmented right percutaneous nephrostomy tube (case 2). (C) Impacted calculus at the right pelvi-ureteric junction with a fragmented nephrostomy tube (case 3). (D) Multiple left renal calculi with a fragmented nephrostomy tube (case 4). |
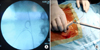 | FIG. 2(A) Intraoperative fluoroscopic image showing a right renal stone and fragmented pigtail nephrostomy tube. Ureteric catheter and antegrade guidewire have been passed (case 5). (B) Intraoperative picture showing the retrieved pigtail nephrostomy tube. Note the breakage at the junction of the coil and straight portion of the tube. |
References
1. Goodwin WE, Casey WC, Woolf W. Percutaneous trocar (needle) nephrostomy in hydronephrosis. J Am Med Assoc. 1955. 157:891–894.
2. Lee WJ, Badlani GH, Smith AD. Percutaneous nephrostomy for endopyelotomy. AJR Am J Roentgenol. 1987. 148:189–192.
3. Zagoria RJ, Dyer RB. Do's and don't's of percutaneous nephrostomy. Acad Radiol. 1999. 6:370–377.
4. Lee WJ. Han MC, Park JH, editors. Percutenaous nephrostomy. Interventional radiology. 1999. Seoul: Ilchokak;591–600.
5. Banner MP, Ramchandani P, Pollack HM. Interventional procedures in the upper urinary tract. Cardiovasc Intervent Radiol. 1991. 14:267–284.
6. el-Faqih SR, Shamsuddin AB, Chakrabarti A, Atassi R, Kardar AH, Osman MK, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991. 146:1487–1491.
7. Marx M, Bettmann MA, Bridge S, Brodsky G, Boxt LM, Richie JP. The effects of various indwelling ureteral catheter materials on the normal canine ureter. J Urol. 1988. 139:180–185.
8. Beiko DT, Knudsen BE, Watterson JD, Cadieux PA, Reid G, Denstedt JD. Urinary tract biomaterials. J Urol. 2004. 171(6 Pt 1):2438–2444.
9. Hwang TK. Percutaneous nephroscopic surgery. Korean J Urol. 2010. 51:298–307.
10. Mardis HK, Kroeger RM, Hepperlen TW, Mazer MJ, Kammandel H. Polyethylene double-pigtail ureteral stents. Urol Clin North Am. 1982. 9:95–101.
11. Zisman A, Siegel YI, Siegmann A, Lindner A. Spontaneous ureteral stent fragmentation. J Urol. 1995. 153(3 Pt 1):718–721.
12. Schulze KA, Wettlaufer JN, Oldani G. Encrustation and stone formation: complication of indwelling ureteral stents. Urology. 1985. 25:616–619.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download