Abstract
Purpose
Tumor size and multiplicity are known to be important prognostic factors in non-muscle-invasive bladder cancer (NMIBC). However, evaluation of accurate tumor size is subjective and difficult. Furthermore, there are limitations to the objectification of tumor volume in the case of multiple lesions. In this study, we investigated the relation between resection weight after transurethral resection of bladder tumor (TURBT) and the prognosis of NMIBC.
Materials and Methods
This was a retrospective analysis of 406 patients diagnosed with pTa or pT1 bladder tumors after TURBT between September 1999 and May 2010. The patient's age, sex, underlying diseases, cancer stage, grade, multiplicity, tumor size, lymphovascular invasion, and resection weight were analyzed in relation to cancer progression and recurrence. The resection weight was weighted after formaldehyde fixation.
Results
The mean follow-up time was 76.9 months (range, 12 to 167 months) in 406 patients diagnosed as having NMIBC. Mean resection weight was 4.5 g (range, 0.1 to 35.0 g). The cancer recurred in 99 patients (24.4%), and disease progression was noted in 30 patients (7.4%). Resection weight was categorized as greater than or less than 2 g by use of receiver operator characteristic curves. Cancer grade (p=0.022) and multiplicity (p=0.043) were significantly related to cancer recurrence in the analysis with Cox's multivariate proportional hazard model. Cancer grade (p=0.001) and resection weight (p=0.018) were related to disease progression.
Bladder cancer is the fourth most common cancer in men and the eighth most common in women [1,2]. Approximately 70% of all patients with bladder cancer have a noninvasive (stage pTa) or lamina propria-invasive (stage pT1) tumor at the time of initial diagnosis [3-5]. Transurethral resection of bladder tumor (TURBT) is performed for the diagnosis and initial treatment of such lesions [6].
For non-muscle-invasive bladder cancer (NMIBC), the probability of recurrence after TURBT at 1 year is 15 to 70% and the probability of progression at 5 years is 7 to 40% [7,8]. Tumor size and multiplicity are known to be important prognostic factors in NMIBC [7-10]. However, in comparison with multiplicity, evaluation of accurate tumor size is subjective and difficult. In particular, there are limitations to determining objective tumor volume in the case of multiple lesions. These limitations are expected to be corrected by the use of tumor weight. Accordingly, we used the weight of the tumor to measure the volume of the tumor. The purpose of the present study was to investigate the relations between resection tumor weight after TURBT and the prognosis of NMIBC.
We retrospectively reviewed the charts of consecutive patients with NMIBC who underwent TURBT at our institution from September 1999 to May 2010. Our inclusion criteria were as follows: 1) pathological stage of Ta, T1 at initial diagnosis; 2) performance of TURBT with an available pathology report for review; 3) documentation of the current status of disease; and 4) ability to check the tumor resection weight in the pathology report. Individuals were excluded if they had evidence of carcinoma in situ in the pathology report, had not received intravesical bacillus Calmette-Guerin (BCG) instillations, or had evidence of distant metastasis (including regional nodal metastasis) at diagnosis [6]. Of the 593 NMIBC patients, 474 (77.9%) were primary. Thirty-five cases with primary carcinoma in situ, 31 cases in which tumor resection weight could not be checked, and 2 cases who did not receive intravesical BCG instillations were excluded from the study. In all, 406 patients met our inclusion criteria. One immediate postoperative intravesical chemotherapy procedure was done among the included patients.
We analyzed the patient's age, sex, underlying diseases, cancer stage (pTa, pT1), grade, multiplicity (more or fewer than 3 lesions), size (more or less than 3 cm), lymphovascular invasion, and resection weight in relation to cancer progression and recurrence. The end-points of our study were recurrence or progression defined as a shift to stage T2, T3, or T4 disease.
All resected specimens were submitted to a department of pathology and fixed in formaldehyde, sectioned, and stained with hematoxylin and eosin. Pathologists evaluated the tissues for tumor stage, grade, pathologic staging, pathologic characteristics, and tumor weight.
Cancer stage was determined by using the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system and histological grade was determined with the 2004 World Health Organization (WHO) grading system. Resected tumor weight was measured after fixation in formaldehyde. We measured only tumor weight, excluding resected deep biopsy and margin weights, which are measures of normal tissue. We then used the probability to plot receiver operating characteristic curves and found an optimal cutoff point of 2 g by maximizing tumor recurrence and progression (Fig. 1). The sensitivity and specificity of this cutoff point were assessed.
Follow-up was performed according to our institution's protocol. Cystoscopy was performed every 3 months for 2 years after TURBT, then every 6 months from 2 to 5 years, and then annually after 5 years. Patients were also examined by physical examination, general laboratory tests, chest X-ray, and urine cytology at each visit for cystoscopy. Abdominal and pelvic cross-sectional imaging was performed annually until 5 years after TURBT. In cases with visible tumor lesions or hyperemic mucosal findings in the bladder by cystoscopy or positive urinary cytological findings, transurethral biopsy or TURBT was carried out to investigate recurrence or progression.
All data were statistically analyzed by using the statistical software SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Estimation of the cumulative distribution of the disease-free interval as a separate factor was calculated according to the Kaplan-Meier method. For multivariate analyses, we used a Cox's proportional hazards regression model. In all tests, p-values<0.05 were considered statistically significant.
Table 1 shows the demographic and clinical characteristics of the TURBT patients; the mean patient age was 64.4 years, and men made up 83.5% of the study population. The median follow-up period was 76.9 months (range, 12 to 167 months) after initial TURBT. Tumor grade was low in 40.6% and high in 59.4%, and tumor stage was pTa in 67.5% and pT1 in 32.5%. Tumor size was larger than 3 cm in 47.3% and smaller than 3 cm in 52.7%. The number of tumors was more than three in 74.6% and fewer than three in 25.4%. Of 406 patients, 99 (24.4%) developed recurrence and 30 (7.4%) progression.
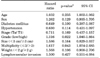
Kaplan-Meier analysis revealed that recurrence was statistically significantly associated with stage (p=0.001), multiplicity (p=0.024), and lymphovascular invasion (p=0.002) (Fig. 2). No statistically significant differences were found for grade or weight. However, multivariate Cox's proportional hazards analysis revealed that disease recurrence was significantly associated with grade (p=0.022) and multiplicity (p=0.043) (Table 2). Stage, tumor size, and tumor weight were not predictors of recurrence. Recurrence risk was 1.6 times higher for tumor multiplicity (more than three) and 1.3 times higher for grade (high grade).
Kaplan-Meier analysis revealed that progression was statistically significant for stage (p=0.013), grade (p=0.002), multiplicity (p=0.001), weight (p=0.017), and lymphovascular invasion (p=0.023) (Fig. 3). No statistically significant differences were found for tumor size. However, multivariate Cox's proportional hazards analysis demonstrated that grade (p=0.001) and tumor weight (p=0.018) were significant prognostic factors of progression (Table 3). Patients with high grade had a 2.6 times higher progression risk than did those with low grade. Tumor weight more than 2 g was the most important prognostic factor because the risk of progression was 4 times higher than that for tumor weight less than 2 g. Patient age, sex, underlying disease, tumor stage, tumor size, multiplicity, and lymphovascular invasion were not statistically significant.
In the management of NMIBC, standard clinicopathological criteria such as multiplicity, tumor size, prior recurrence rate, T stage, tumor grade, and presence of concomitant CIS have been used to assess the risk of recurrence and progression [11,12]. In addition, various environmental factors are associated with the carcinogenesis of bladder cancer, and the degree of exposure to these environmental carcinogens may vary in different countries and cultures [13]. Susceptibility to carcinogens also depends on genetic background, and such differences could contribute to the carcinogenesis of bladder cancer and the subsequent discrepancy in the incidence of recurrence or progression [13]. Most of all, it is known that the most important risk factor for progression is grade, not stage [9,14]. Prognosis also correlates with tumor size, multiplicity, papillary versus sessile configuration, presence or absence of lymphovascular invasion, and status of the remaining urothelium [14,15].
Although tumor size and multiplicity are generally considered prognostic factors in patients with NMIBC after TURBT [16,17], in some studies, the conclusions made about prognostic values were based on univariate analysis alone. In our study, in the multivariate analysis, tumor size was not a prognostic factor for recurrence and neither tumor size nor multiplicity was a prognostic factor for progression. In the univariate analysis, however, tumor size and multiplicity were prognostic factors for progression. As mentioned earlier, several factors such as environmental carcinogens, genetic backgrounds, and different countries and cultures may account for this discrepancy.
The most commonly used staging system is the TNM system proposed by the AJCC [18]. In the TNM system, except for bladder cancer, T stage commonly includes tumor size and multiplicity. The major factors in staging of bladder cancer, however, are depth of invasion into the bladder wall and the degree of differentiation (pathologic grade) of the tumor. Tumor size and multiplicity are not included in the TNM staging of bladder cancer. Nevertheless, several studies have determined that tumor size and multiplicity are significant predictors of cancer recurrence and progression.
Staging with cystoscopy is most suited for superficial tumors [19]. TURBT is performed both to remove all visible tumors and to provide specimens for pathologic examination to determine stage and grade. However, tumor size and multiplicity measured after TURBT and cystoscopy do not reflect the bladder tumor volume. Generally, tumor size measured as the diameter of the tumor and multiplicity do not correspond with tumor volume for tumor shapes such as sessile and papillary. We expected that these limitations might be corrected by the use of tumor volumes in the present study. Prediction of recurrence and progression with the use of tumor volumes was thought to be more suitable than the use of size and multiplicity. The weight of the tumor was used to measure the volume of the tumor in this study. Also, any factors already known to be related to recurrence and progression, including size and multiplicity, were included in the analysis.
A new finding in our study was that tumor weight increased the risk of progression. Our study showed that tumor weight predicts disease progression in patients with diagnosed NMIBC (p=0.018). The role of tumor weight in the progression of bladder cancer has not been studied by others; only tumor size and multiplicity have been studied previously.
Our prognostic factors for recurrence were grade and multiplicity (Table 2). Unlike in the other studies, in our study, grade was a prognostic factor for recurrence but tumor stage was not. We also proved a higher recurrence risk for multiple tumors. The significance of tumor size is controversial because it was not a prognostic factor in our study or in others [12,13,15,16] but was significant in some studies [13,14,19].
In our study, the prognostic factors for progression were grade and, most importantly, tumor weight (Table 3). The fact that grade was a prognostic factor for progression agrees with the findings of others. Unlike in other studies, however, tumor stage, multiplicity, and tumor size were not prognostic factors for progression in our study. This discrepancy might be attributed to the fact that progression was considered as a shift to stage T2, T3, or T4. Unlike in other studies, neither node invasion nor metastasis was considered in our definition of tumor progression.
There were several limitations to our study. Our results were based on a retrospective analysis from a single institution. It is thought that measurement of tumor volume itself has limitations. Electrical and thermal injuries during TURBT are inevitable; hence, accurate measurement of tumor specimens was not possible. Also, although the tumor base was excluded from the weight measurement when cancer-negative, and NMIBC without pTIs was defined as an inclusion criterion to measure accurate tumor weight itself, we expect limitations. Furthermore, because of the long enrollment period of the study, there were changes in the WHO grading system and TNM staging system during this period. Although any changes in grade and stage were corrected for, different periods of follow-up already completed before the system changed were impossible to be corrected for.
After primary NMIBC has been treated with TURBT, it can be assumed that not only primary tumor multiplicity, but also grade and tumor weight are important determinants of the clinical course of NMIBC. Furthermore, in the multivariate analysis, the resection weight after TURBT (tumor weight) had statistical significance for the progression of NMIBC. Therefore, we may consider the resection weight after TURBT in the management of NMIBC.
Figures and Tables
FIG. 1
Receiver-operating characteristic (ROC) curve (tumor weight). Points (black arrow) on the ROC curve represent the possibility levels generated from the logistic regression analysis that was used to select the optimal cutoff point. A predicted probability of 2 provided a sensitivity of 70.5% and a specificity of 57.0%.

FIG. 2
Kaplan-Meier plot for recurrence comparing Ta and T1 tumors (p=0.001), grades low and high (p=0.125), tumors greater than and less than 3 cm (p=0.190), multiplicity more than 3 and less than 3 (p=0.024), tumors greater than and less than 2 g (p=0.184), and lymphovascular invasion (p=0.002).

FIG. 3
Kaplan-Meier plot for progression comparing Ta and T1 tumors (p=0.013), grades low and high (p=0.002), tumors greater than and less than 3 cm (p=0.166), multiplicity more than 3 and less than 3 (p=0.001), tumors greater than and less than 2 g (p=0.017), and lymphovascular invasion (p=0.023).

References
1. National Cancer Center [internet]. The statistics report: the incidence of cancer on 2003-2005 and the survival rate on 1993-2005. c2012. cited 2009 Apr 1. Goyang: National Cancer Center;Available from: http://www.ncc.re.kr.
2. Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005. 55:10–30.
3. Kim YJ, Moon KH, Kim KR, Kim CS. Significance of T1 substaging on the recurrence and progression of bladder cancer. Korean J Urol. 2000. 41:1323–1328.
4. Kwon S, Park CM, Kim HG, Gang GH, Song JS, Park JY. Prognostic significance of the tumor configuration in superficial bladder tumor. Korean J Urol. 2006. 47:237–243.
5. Reading J, Hall RR, Parmar MK. The application of a prognostic factor analysis for Ta.T1 bladder cancer in routine urological practice. Br J Urol. 1995. 75:604–607.
6. Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007. 178:2314–2330.
7. Legrand G, Soliman H, Dubosq F, Verine J, Desgrandchamps F, de The H, et al. Prevalence and spectrum of microsatellite alterations in nonmuscle invasive bladder cancers. Am J Cancer Res. 2011. 1:595–603.
8. Kurth KH, Denis L, Bouffioux C, Sylvester R, Debruyne FM, Pavone-Macaluso M, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer. 1995. 31A:1840–1846.
9. Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodriguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol. 2000. 163:73–78.
10. Ali-El-Dein B, Sarhan O, Hinev A, Ibrahiem el-HI, Nabeeh A, Ghoneim MA. Superficial bladder tumours: analysis of prognostic factors and construction of a predictive index. BJU Int. 2003. 92:393–399.
11. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006. 49:466–475.
12. Barocas DA, Clark PE. Bladder cancer. Curr Opin Oncol. 2008. 20:307–314.
13. Hirao Y, Kim WJ, Fujimoto K. Environmental factors promoting bladder cancer. Curr Opin Urol. 2009. 19:494–499.
14. Jones JS, Larchian WA. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Non-muscle-invasive bladder cancer (Ta, T1, and CIS). Campbell-Walsh urology. 2012. 10th ed. Philadelphia: Saunders;2335–2354.
15. Kunju LP, You L, Zhang Y, Daignault S, Montie JE, Lee CT. Lymphovascular invasion of urothelial cancer in matched transurethral bladder tumor resection and radical cystectomy specimens. J Urol. 2008. 180:1928–1932.
16. Lee SE, Jeong IG, Ku JH, Kwak C, Lee E, Jeong JS. Impact of transurethral resection of bladder tumor: analysis of cystectomy specimens to evaluate for residual tumor. Urology. 2004. 63:873–877.
17. Cho KS, Seo HK, Joung JY, Park WS, Ro JY, Han KS, et al. Lymphovascular invasion in transurethral resection specimens as predictor of progression and metastasis in patients with newly diagnosed T1 bladder urothelial cancer. J Urol. 2009. 182:2625–2630.
18. Chang SS, McKiernan JM, Amin M, Bochner BH, Campbell S, Gospodarowicz MK, et al. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. Urinary bladder. AJCC cancer staging manual. 2010. 7th ed. New York: Springer;497–505.
19. Dighe MK, Bhargava P, Wright J. Urinary bladder masses: techniques, imaging spectrum, and staging. J Comput Assist Tomogr. 2011. 35:411–424.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download