Abstract
Purpose
We aimed to ascertain the effects of performing extended pelvic lymph node dissection (PLND) on the duration of surgery, morbidity, and the number of lymph nodes removed when the dissection was performed before or after radical cystectomy (RC).
Materials and Methods
We used the database of our previous prospective multicenter study. A total of 118 patients underwent RC and extended PLND. Of the 118 patients, 48 (40.7%) underwent extended PLND before RC (group 1) and 70 (59.3%) underwent extended PLND after RC (group 2). The two groups were compared for extended PLND time, RC time, and total operation times, per operative morbidity, and the total numbers of lymph nodes removed.
Results
Clinical and pathologic characteristics were comparable in the two groups (p>0.05). The mean RC time and mean total operation times were significantly shorter in group 1 than in group 2 (p<0.001). The mean number of lymph nodes removed was 27.31±10.36 in group 1 and 30.87±8.3 in group 2 (p=0.041). Only at the presacral region was the mean number of lymph nodes removed significantly fewer in group 1 than in group 2 (p=0.001). Intraoperative and postoperative complications and drain withdrawal time were similar in both groups (p=0.058, p=0.391, p=0.613, respectively).
Conclusions
When extended PLND was performed before RC, the duration of RC and consequently the total duration of the operation were significantly shorter than when extended PLND was performed after RC. Practitioners may consider performing extended PLND before RC and rechecking the presacral area for additional lymph nodes after RC, particularly in elderly patients with high co-morbidity for whom the duration of surgery matters.
Radical cystectomy (RC) with pelvic lymph node dissection (PLND) is the standard of care for muscle-invasive bladder cancer [1]. Previous studies have demonstrated that extended PLND ensures more accurate staging and prognostic information and has therapeutic benefits in a select subset of patients with positive and even in patients with negative lymph nodes [2-6]. Especially during the past decade, numerous articles have been published focusing on issues such as the extended PLND technique (open, laparoscopic, or robotic-assisted), indications and restrictions, effects on survival and morbidity, the minimum number of lymph nodes to be removed to elicit prognostic and therapeutic benefit, the reliability of examination of frozen sections from the obturator fossa, and the impact on lymphadenectomy margins, lymph node density, and submission of pelvic lymph nodes from different sites separately or en bloc [3,4,6-19]. However, to the best of our knowledge, no study had been conducted to evaluate the effects of the timing of lymphadenectomy during RC, that is, before or after. Therefore, to evaluate this issue, we used the database of our previous prospective multicenter study in which RC and extended PLND was performed [14]. The aim of this study was to ascertain the effects of performing extended PLND before or after RC on the duration of operation, morbidity, and the number of lymph nodes removed.
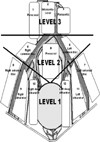
In our previous prospective study, 15 surgeons in 13 centers performed open RC and extended PLND on 118 patients diagnosed with bladder cancer [14]. The World Health Organization 1998 classification was used for histopathological typing and grading [20]. Pathologic staging was assigned according to the 2002 tumor-node-metastasis classification [21]. All patients underwent extended PLND, for which margins were determined previously by a protocol [14]. The tissues removed from 12 distinct lymph node regions (Fig. 1) were sent to pathological examination separately.
Of the 118 patients, 48 (40.7%) underwent extended PLND before RC (group 1), whereas 70 (59.3%) underwent extended PLND after RC (group 2). The two groups were compared for extended PLND time, RC time, and total operation (RC+extended PLND without urinary diversion) times, per operative morbidity; the number of lymph nodes removed; and the number of lymph nodes removed from three lymphadenectomy levels (Fig. 1) as described by Leissner et al. [19] and which were also used in our previous study [14].
Statistical analyses were performed by using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). Student's t-test or the Mann Whitney U test was used to compare the two groups for continuous variables and the chi-square test was used for categorical variables. Only p-values<0.05 were considered statistically significant.
Mean patient age was 61.09±9.755 years (range, 27 to 82 years). Of the 118 patients, 107 were men and 11 were women. The clinical and pathological characteristics of the patients are shown in Table 1. Histological cell type, grade, and clinical and pathological tumor and node stage were similar in the two groups (all p-values>0.05).
The mean RC, extended PLND, and total operation times of the two groups are presented in Table 2. The mean extended PLND time was similar in both groups (p=0.160). However, the mean RC time and mean total operation times were significantly shorter in group 1 than in group 2 (p<0.001) (Table 2).
The mean number of lymph nodes removed was 27.31±10.36 in group 1 and 30.87±8.3 in group 2 (p=0.041). Table 3 shows the mean and median number of lymph nodes removed from the three distinct lymphadenectomy levels (Fig. 1) described by Leissner et al. [19] for both groups in a comparative manner. The number of lymph nodes removed from level 2 was significantly fewer in group 1 than in group 2 (p=0.005), whereas the numbers of lymph nodes removed from level 1 and level 3 were similar in both groups (p=0.083 and 0.261, respectively) (Table 3). At level 2, where the number of lymph nodes removed was significantly fewer in group 1 than in group 2, there were 3 lymph node regions: region 4 (right common iliac), region 6 (left common iliac), and region 8 (presacral) (Fig. 1). Of 118 patients, in 32 patients at least one region on level 2 was reported to have no pathological lymph nodes. The distribution of the regions in the patients was as follows: region 8 in 14 patients, region 6 in 5 patients, region 4 in 3 patients, regions 4 and 8 in 5 patients, regions 6 and 8 in 3 patients, regions 4 and 6 in 1 patient, and regions 4, 6, and 8 in 1 patient. For both groups, the mean and median numbers of lymph nodes removed from these three lymphadenectomy regions at level 2 are shown in Table 4. Two patients in group 1 and two patients in group 2 had positive lymph nodes at region 8 (p=0.699). The mean lymph node density values for region 8 were 0.368±0.175 and 0.328±0.179 in groups 1 and 2, respectively (p=0.562).
Intraoperative arterial or venous injury emerged and was repaired in 12 of 118 patients, 8 in group 1 and 4 in group 2. Of these 12 patients, 7 required blood transfusion, 4 in group 1 and 3 in group 2. The frequency of intraoperative complications was comparable between the two groups (p=0.058). Postoperative complications occurred in 27 patients, including prolonged lymphatic drainage in 20 (10 patients in each group), deep vein thrombosis in 3 (all in group 2), prolonged ileus in 3 (2 patients in group 1 and one patient in group 2), and late hemorrhage in 1 patient (in group 1). The frequency of postoperative complications was comparable between the two groups (p=0.391).
The mean surgical drain withdrawal time was 11.62 days for all patients included in the study. It was 12.02 days in group 1 and 11.34 days in group 2. Mean surgical drain withdrawal time was similar in both groups (p=0.613).
Lymph node metastases are found in approximately one third to one fourth of patients with bladder cancer who undergo RC and PLND and are the most important prognostic factor in these patients [4,14,22-24]. The importance of the extent of PLND for bladder cancer has become clearer with increasing evidence supporting improved outcomes with more extensive dissections [2,5,6]. However, no recommendation before now has been made with regard to performing extended PLND before or after RC. Some surgeons prefer to perform extended PLND before RC, whereas others prefer to perform it after RC. Both options have advantages and disadvantages. The potential advantages of performing extended PLND before RC are clear visualization of the pedicles and clarified tissue planes. Thus, RC can be performed more rapidly and without significant blood loss. The disadvantages of performing extended PLND before RC result from the narrow space in the pelvis, especially in patients with large solid bladder tumors, which makes the dissection difficult during PLND. On the other hand, performing extended PLND after RC has the advantage of a wide working area in the narrow pelvic cavity and, because the blood vessels are clearly identified, PLND can be performed easily.
However, many studies have suggested that the morbidity associated with extended PLND is comparable with that of a more limited approach [4,9,10]. To our knowledge, however, none of the studies reported evaluated the effect of the timing of extended PLND on the operative morbidity. In our study, we showed that performing extended PLND before or after RC did not result in differences with regard to morbidity. Thereby, considering morbidity, the timing of extended PLND, before or after RC, has no importance.
Many studies have reported that extended PLND takes up to 60 minutes longer than a lymphadenectomy that cranially ends at the level of the iliac arteries [4,9,10,19]. Hence, in daily practice, some surgeons avoid extended PLND in patients with high comorbidity for whom operative time is important. In our study, the mean extended PLND time was 87.08 minutes in group 1 (pre-RC group) and 79.71 minutes in group 2 (post-RC group). Performing extended PLND before or after RC makes an 8-minute difference in the duration of extended PLND, which is statistically insignificant. Therefore, the timing of extended PLND does not result in any significant differences in the duration of extended PLND. In contrast, when we consider the mean RC time, it was shortened by 40 minutes when RC was performed after extended PLND instead of before extended PLND, which was statistically significant. The total mean operation time was also shortened by 33 minutes in group 1 (pre-RC group) compared with group 2 (post-RC group) and this was also statistically significant. These data indicate that performing extended PLND before RC shortens the total operation time by shortening particularly the RC time. Because extended PLND before RC fully skeletonizes the pelvic vascular structures, isolation and control of pedicles during RC is easier, which might be an explanation for the shortening of RC time. Thereby, because there is no difference in morbidity, it can be recommended that extended PLND be performed before RC because the total operation time is shorter than when extended PLND is performed after RC.
Several studies have indicated that the number of lymph nodes removed has prognostic significance in both lymph-node-positive and lymph-node-negative patients [2-4,11,25,26]. In most of the studies and in the European Association of Urology 2010 guidelines on bladder cancer, removal of more than 15 lymph nodes was postulated to be sufficient for the evaluation of lymph node status as well as beneficial for overall survival [4,12,27,28]. In another collaborative review article, it was reported that removal of ≥20 lymph nodes per patient reflects the mean number of nodes that are removed during a meticulous PLND [26]. In our study, we also evaluated the effect of performing extended PLND before or after RC on the number of lymph nodes removed. The mean number of lymph nodes removed during extended PLND before (group 1) and after (group 2) RC was 27.3 and 30.9, respectively, which was a statistically significant difference (p=0.041). Although the number of lymph nodes removed was slightly reduced when extended PLND was performed before RC, it was much higher than 15 or 20, which are the values accepted as cutoffs for improving staging, prognosis, and survival [4,26-28]. In our study, in order to determine whether the mean number of lymph nodes removed at one level made a difference between the two groups with regard to the mean total number of lymph nodes removed, the mean numbers of lymph nodes removed from 3 lymphadenectomy levels (Fig. 1) were compared. Regarding the number of lymph nodes removed, we found a significant difference between the two groups only at level 2. We then tried to determine whether the mean number of lymph nodes removed was significantly different in any 1 of the 3 regions (right common iliac, left common iliac, and presacral) at level 2. Regarding the mean number of lymph nodes removed, we found a significant difference between the two groups only at region 8 (presacral region). At this region, a significantly lower mean number of lymph nodes was removed in group 1 than in group 2. However, the number of patients with positive nodes and mean lymph node density values were found to be similar in the two groups. Nevertheless, in patients who had extended PLND before RC, it may be advisable to recheck the presacral area for additional lymph nodes after cystectomy. The statistically significant decrease in the mean number of lymph nodes removed from the presacral region by performing extended PLND before RC can be explained in several ways. To perform lymphadenectomy at the presacral region, initial mobilization of the sigmoid mesentery is essential and this process may become easier after cystectomy. Moreover, dissection of the proximal common iliac artery and presacral region become easier because the ureters have already been cut, mobilized, and taken away from the surgical area during RC.
There are several limitations to this study. First, this was not a randomized clinical study and therefore it bears all the biases inherent with such a study. It could be argued that all of the differences were due to differences in the experience of the surgeons. However, such a magnitude of difference in the technical skills of the surgeons from major centers participating in this study is unlikely. Second, there were no criteria set up front to evaluate the surgical quality of this complicated surgery. On the other hand, the prospective data collection, which was based on a standard protocol, might have decreased the possible biases.
Performing extended PLND before RC shortens the total operation time in comparison with performing extended PLND after RC by shortening the RC time in particular and does not lead to any additional morbidity. On the other hand, when extended PLND is performed before RC, particularly owing to the difficult dissection of the presacral region, the total number of lymph nodes removed is reduced compared with when extended PLND is performed after RC. It can be recommended that extended PLND be performed before RC and that the presacral area be rechecked for additional lymph nodes after cystectomy, particularly in patients with high comorbidity for whom the operation time is important.
Figures and Tables
ACKNOWLEDGEMENTS
The authors thank Levent Turkeri, Suleyman Ataus, Recep Buyukalpelli, Ugur Altug, Ugur Kuyumcuoglu, Ozdal Dillioglugil and Hayrettin Şahin for participating with their patients in the trial.
References
1. Huang GJ, Stein JP. Open radical cystectomy with lymphadenectomy remains the treatment of choice for invasive bladder cancer. Curr Opin Urol. 2007. 17:369–375.
2. Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002. 167:1295–1298.
3. Konety BR, Joslyn SA, O'Donnell MA. Extent of pelvic lymphadenectomy and its impact on outcome in patients diagnosed with bladder cancer: analysis of data from the Surveillance, Epidemiology and End Results Program data base. J Urol. 2003. 169:946–950.
4. Leissner J, Hohenfellner R, Thüroff JW, Wolf HK. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int. 2000. 85:817–823.
5. Stein JP, Cai J, Groshen S, Skinner DG. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol. 2003. 170:35–41.
6. Dhar NB, Klein EA, Reuther AM, Thalmann GN, Madersbacher S, Studer UE. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol. 2008. 179:873–878.
7. Guru KA, Sternberg K, Wilding GE, Tan W, Butt ZM, Mohler JL, et al. The lymph node yield during robot-assisted radical cystectomy. BJU Int. 2008. 102:231–234.
8. Ghazi A, Zimmermann R, Al-Bodour A, Shefler A, Janetschek G. Optimizing the approach for lymph node dissection during laparoscopic radical cystectomy. Eur Urol. 2010. 57:71–78.
9. Poulsen AL, Horn T, Steven K. Radical cystectomy: extending the limits of pelvic lymph node dissection improves survival for patients with bladder cancer confined to the bladder wall. J Urol. 1998. 160(6 Pt 1):2015–2019.
10. Brössner C, Pycha A, Toth A, Mian C, Kuber W. Does extended lymphadenectomy increase the morbidity of radical cystectomy? BJU Int. 2004. 93:64–66.
11. Herr HW, Faulkner JR, Grossman HB, Natale RB, deVere White R, Sarosdy MF, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004. 22:2781–2789.
12. Dangle PP, Gong MC, Bahnson RR, Pohar KS. How do commonly performed lymphadenectomy templates influence bladder cancer nodal stage? J Urol. 2010. 183:499–503.
13. Adsan O, Baltaci S, Cal C, Buyukalpelli R, Ugurlu O, Bozlu M, et al. Reliability of frozen section examination of external iliac, hypogastric, and obturator lymph nodes during radical cystectomy: a multicenter study. Urology. 2007. 69:83–86.
14. Baltaci S, Adsan O, Ugurlu O, Aslan G, Can C, Gunaydin G, et al. Reliability of frozen section examination of obturator lymph nodes and impact on lymph node dissection borders during radical cystectomy: results of a prospective multicentre study by the Turkish Society of Urooncology. BJU Int. 2011. 107:547–553.
15. Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol. 2003. 169:943–945.
16. Wiesner C, Salzer A, Thomas C, Gellermann-Schultes C, Gillitzer R, Hampel C, et al. Cancer-specific survival after radical cystectomy and standardized extended lymphadenectomy for node-positive bladder cancer: prediction by lymph node positivity and density. BJU Int. 2009. 104:331–335.
17. Stein JP, Penson DF, Cai J, Miranda G, Skinner EC, Dunn MA, et al. Radical cystectomy with extended lymphadenectomy: evaluating separate package versus en bloc submission for node positive bladder cancer. J Urol. 2007. 177:876–881.
18. Bochner BH, Herr HW, Reuter VE. Impact of separate versus en bloc pelvic lymph node dissection on the number of lymph nodes retrieved in cystectomy specimens. J Urol. 2001. 166:2295–2296.
19. Leissner J, Ghoneim MA, Abol-Enein H, Thuroff JW, Franzaring L, Fisch M, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J Urol. 2004. 171:139–144.
20. Epstein JI, Amin MB, Reuter VR, Mostofi FK. Bladder Consensus Conference Committee. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol. 1998. 22:1435–1448.
21. Sobin LH, Wittekind C. TNM classification of malignant tumours. 2002. 6th ed. New York: Wiley-Liss.
22. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001. 19:666–675.
23. Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006. 176:2414–2422.
24. Dorin RP, Skinner EC. Extended lymphadenectomy in bladder cancer. Curr Opin Urol. 2010. 20:414–420.
25. Holmer M, Bendahl PO, Davidsson T, Gudjonsson S, Mansson W, Liedberg F. Extended lymph node dissection in patients with urothelial cell carcinoma of the bladder: can it make a difference? World J Urol. 2009. 27:521–526.
26. Karl A, Carroll PR, Gschwend JE, Knuchel R, Montorsi F, Stief CG, et al. The impact of lymphadenectomy and lymph node metastasis on the outcomes of radical cystectomy for bladder cancer. Eur Urol. 2009. 55:826–835.
27. Fleischmann A, Thalmann GN, Markwalder R, Studer UE. Extracapsular extension of pelvic lymph node metastases from urothelial carcinoma of the bladder is an independent prognostic factor. J Clin Oncol. 2005. 23:2358–2365.
28. Studer UE, Collette L. Morbidity from pelvic lymphadenectomy in men undergoing radical prostatectomy. Eur Urol. 2006. 50:887–889.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download