Abstract
Malignant transformation of urachal adenoma is exceedingly rare, with intestinal metaplasia as the most common contributing mechanism. It is recommended that a urachal adenoma be regarded as a pre-malignant condition and be subject to endoscopic surveillance. A local en block excision of the tumor mass with urachalectomy and umbilectomy results in possible long-term survival. The median survival after platinum-based chemotherapy is limited for patients with extravesical disease. Here we report a case of synchronous urothelial carcinoma of the bladder and urachal adenoma that transformed into adenocarcinoma.
Malignant transformation of a urachal adenoma into an adenocarcinoma is exceedingly rare. Here we report a case of synchronous urothelial carcinoma of the bladder and urachal adenoma with subsequent malignant transformation.
An 86-year-old female was diagnosed with superficial, low-grade, non-muscle-invasive urothelial carcinoma of the bladder in 1996. She had past medical histories of multiple sclerosis, thoracic aortic aneurysm, and supraventricular tachycardia. After an initial transurethral resection (TUR), she remained asymptomatic and had no tumor recurrence until October 2009. A single urothelial carcinoma situated near the left ureteric orifice was noted during a routine flexible cystoscopy. An incidental 2.5-cm mucus-secreting diverticulum at the dome of the bladder was also noted during the same procedure. A cold cup biopsy of the diverticulum wall was planned to occur simultaneously with a TUR of the bladder tumor. The TUR of the bladder tumor was performed and the histology confirmed a low-grade, non-muscle-invasive urothelial carcinoma. The bladder diverticulum was again visualized at the dome of the bladder during the procedure. Cold cup biopsies of the wall of the diverticulum revealed evidence of tubulovillous adenoma, possibly of urachal origin. Clinically, the patient had no palpable suprapubic mass and a triple-phase computed tomography (CT) scan of the abdomen was normal. Since then, the patient had regular surveillance cystoscopy in accordance with the guidelines on TaT1 (non-muscle-invasive) bladder cancer by the European Association of Urology. In 2011, the patient was again noted to have microscopic hematuria. She had no palpable mass on examination. The bladder diverticulum containing the urachal adenoma was again visualized.
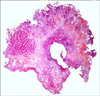
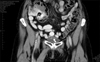
An endoscopic resection of the urachal adenoma was performed and macroscopic clearance of the tumor was achieved. The histology of the specimen revealed a moderately differentiated adenocarcinoma, enteric subtype, with evidence of lamina propria infiltration (Figs. 1, 2). A staging CT scan showed a localized urachal lesion without extravesical extension. The umbilicus and the lymph nodes were unremarkable on the CT scan (Fig. 3).
A diagnosis of urachal adenocarcinoma was made. After careful consideration, taking into account the patient's age, co-morbidities, and the lack of radiological evidence of extravesical disease, a partial cystectomy and urachalectomy was planned. The intravesical approach was used initially to identify the macroscopic extent of the tumor with a 1-cm margin marked out with a Barnes knife and with the bladder full. Laparotomy was then performed with excision of the urachus from the base of the umbilicus down to and including the lesion in the bladder as previously outlined. Histology of the resected specimen showed no residual adenocarcinoma and clear resection margins.
A urachal lesion can be benign or malignant. Benign lesions are most commonly adenomas. Of the malignant lesions, over 80% are adenocarcinoma with enteric, mucinous, and signet ring subtypes, and the rest consist of sarcoma, urothelial carcinoma, and squamous cell carcinoma. About 69% of adenocarcinoma produces mucus and 15% does not [1]. Overall, the incidence of urachal carcinoma is 0.07 to 2% [1]. A patient with urachal adenocarcinoma often presents with macroscopic hematuria. The mean age of presentation is 65 years [1]. Urachal tumors are situated at the junction of the urachus and the bladder. A CT scan of the abdomen may display tumor calcification and umbilical extension with extravesical disease. The location of the tumor at the bladder dome, the sharp demarcation between the tumor and the normal urothelium, the presence of urachal remnants, and the absence of cystitis glandularis and cystitis cystic are diagnostic criteria for urachal neoplasm [2].
Many staging systems have been proposed for urachal tumors. However, no system stands out given the paucity of urachal tumors. The recent reclassification of urachal tumors by the Mayo clinic has simplified the previously known Sheldon system [1,3] The Mayo staging system divides urachal tumors into four stages: stage I, tumor confined to urachus and bladder; stage II, tumor extending beyond urachus and bladder; stage III, tumor infiltration of regional nodes; and stage IV, tumor infiltration of non-regional nodes and distant sites. Most of the tertiary institutions adopt a multi-modal approach when treating patients with a urachal tumor. A local en bloc excision of the tumor mass with urachalectomy and umbilectomy results in possible long-term survival. The median survival after platinum-based chemotherapy is limited for patients with extra-vesical disease [4].
Intestinal metaplasia is the most common mechanism causing the transformation of a urachal adenoma into adenocarcinoma. Only 30 cases of villous adenoma have been found outside the large bowel, and only 4 cases of adenocarcinoma arising within a villous adenoma have been reported [5-7]. The hypothesis was that a urachal tubulovillous adenoma shares similar oncogenes and tumor suppressor protein such as p53 with colonic tubulovillous adenoma. The progression from colonic adenoma to adenocarcinoma is genetically well characterized and thus may be applicable in urachal tumors. Urachal adenoma and adenocarcinoma may in fact co-exist in a solitary lesion, representing a different spectrum of tumorigenesis. A cold cup biopsy of a sizable urachal lesion can hence be falsely reassuring. It is recommended that a urachal adenoma be regarded as a premalignant condition and be subject to endoscopic surveillance. Synchronous low-grade, non-muscle-invasive urothelial carcinoma and urachal adenoma is rare and there is no known genetic link between the two malignancies.
The optimal management of synchronous urothelial carcinoma and urachal adenoma is not known. The potential for malignant transformation of the urachal adenoma into adenocarcinoma proposes a unique clinical challenge. Regular endoscopic surveillance of the urachal adenoma should be mandated; however, a protocol is difficult to formulate given the rarity of the urachal lesion. One needs to weigh the rarity of the disease against its malignant potential when making a clinical decision.
A partial cystectomy with en bloc resection of urachal adenocarcinoma seems to be the widely accepted surgical approach for localized urachal adenocarcinoma. A study by Memorial Sloan Kettering Cancer Center showed a survival rate of 93% at 5 years with partial cystectomy [8]. A study by the Mayo clinic showed a median survival of 10.8 years for stage I/II disease and only 1.3 years for stage IV disease [4]. This compares similarly with the 5-year survival data for T1 high-grade urothelial carcinoma after radical cystectomy and favorably with the 5-year survival data for muscle-invasive urothelial carcinoma [9]. We recommend the use of the European Association of Urology urothelial carcinoma endoscopic surveillance protocol for a solidary urachal adenoma and also in the setting of a synchronous low-grade, superficial urothelial carcinoma with a urachal adenoma.
In conclusion, synchronous bladder urothelial carcinoma and urachal adenoma is rare. Clinicians need to be aware of the premalignant potential of urachal adenoma, and an endoscopic surveillance program should be instigated. The late adenoma to adenocarcinoma transformation should be managed with partial cystectomy and en bloc resection of the urachus and umbilicus in well-selected patients.
Figures and Tables
References
1. Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant urachal lesions. J Urol. 1984. 131:1–8.
2. Paras FA Jr, Maclennan GT. Urachal adenocarcinoma. J Urol. 2008. 180:720.
3. Ashley RA, Inman BA, Sebo TJ, Leibovich BC, Blute ML, Kwon ED, et al. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer. 2006. 107:712–720.
4. Molina JR, Quevedo JF, Furth AF, Richardson RL, Zincke H, Burch PA. Predictors of survival from urachal cancer: a Mayo Clinic study of 49 cases. Cancer. 2007. 110:2434–2440.
5. Assor D. A villous tumor of the bladder. J Urol. 1978. 119:287–288.
6. Lucas DR, Lawrence WD, McDevitt WJ. Mucinous papillary adenocarcinoma of the bladder arising within villous adenoma of urachal remnants. An immuno-histochemical and ultrastructural study. J Urol Pathol. 1994. 2:173–182.
7. Mazzucchelli R, Scarpelli M, Montironi R. Mucinous adenocarcinoma with superficial stromal invasion and villous adenoma of urachal remnants: a case report. J Clin Pathol. 2003. 56:465–467.
8. Herr HW, Bochner BH, Sharp D, Dalbagni G, Reuter VE. Urachal carcinoma: contemporary surgical outcomes. J Urol. 2007. 178:74–78.
9. Stenzl A, Cowan NC, De Santis M, Jakse G, Kuczyk MA, Merseburger AS, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009. 55:815–825.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download