Abstract
Purpose
The prostatic urethra is a bent tube, and the clinical significance of the prostatic urethral angle (PUA) was recently reported. We investigated the statistical significance of an increased PUA on the International Prostate Symptom Score (IPSS) in men with benign prostatic hyperplasia (BPH)/lower urinary tract symptom (LUTS).
Materials and Methods
A retrospective analysis was done of 270 men with BPH/LUTS from July 2009 to June 2011. Prostate volume, PUA, and intravesical prostatic protrusion (IPP) were measured by transrectal ultrasonography (TRUS). The IPSS was analyzed separately as storage and voiding symptom score. In order to minimize the effect of prostate size on voiding, patients with prostate size over 40 ml were excluded.
Results
The mean age was 62.0±9.3 years. The mean prostate volume was 29.0±5.5 ml (range, 20 to 40 ml), and median PUA and IPP were 34° (range, 12 to 52°) and 1.7 mm (range, 0 to 5.3 mm), respectively. The mean IPSS, mean IPSS-ss, and mean IPSS-vs were 19.0±8.2, 7.3±4.0, and 11.6±5.5, respectively. The prostate volume had no statistically significant correlation with IPSS, IPSS-ss, or IPSS-vs. IPP had a statistically significant correlation with IPSS (p<0.001), IPSS-ss (p<0.001), and IPSS-vs (p<0.001). PUA had no statistically significant correlation with IPSS or IPSS-ss. However, PUA had a significant correlation with IPSS-vs (p=0.047). Comparing a higher PUA (≥34°) with a lower PUA (<34°), patients with a higher PUA had a higher IPSS (p=0.001) and a higher IPSS-vs (p=0.001). There was no significant difference in IPSS-ss, prostate volume, or PSA between the two groups.
Benign prostatic hyperplasia (BPH) is one of the most common problems faced by aging men and can be associated with bothersome lower urinary tract symptoms (LUTS). It is well known that BPH can affect quality of life by interfering with normal daily activities and sleep patterns. Currently, evaluation for the treatment of BPH includes digital rectal examination, the International Prostate Symptom Score (IPSS), serum prostate-specific antigen levels (PSA), and uroflowmetry with postvoid residual urine (PVR). Urodynamic study and transrectal ultrasonography (TRUS) are done in special circumstances [1].
Several studies have reported the importance of anatomical factors in evaluating men with BPH. One of these is a recently reported hypothesis stating that because the prostatic urethra is a bent tube, the prostatic urethral angle (PUA) may be a causal factor for BPH pathogenesis and LUTS [2,3]. Also, the IPSS, which was originally developed by the World Health Organization (WHO), has been widely adopted and used as a reliable method for detecting and categorizing the severity of LUTS.
In the present study, we investigated whether an increased PUA significantly affects the IPSS in men with BPH.
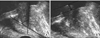
Retrospective analysis was done from July 2009 to June 2011 of 270 men with BPH. These patients underwent a detailed clinical evaluation including a medical history, digital rectal examination, IPSS, frequency-volume chart, urinalysis, PSA, uroflowmetry with PVR, and TRUS. Prostate volume, PUA, and IPP were measured on the image findings of TRUS. PUA was calculated on the midsagittal plane of the prostate as described in a previous report and was recorded from 0 to 90° (Fig. 1A) [3]. IPP was measured as the shortest distance between the protruded end of the prostate and the bladder base on the bladder neck in the sagittal plane, which reflects the maximum longitudinal length of the prostate as suggested by Nose et al. [4] (Fig. 1B).
The IPSS was recorded and separated as the storage symptom (IPSS-ss) and voiding symptom (IPSS-vs) subscores. Patients with a urologic tumor, neurogenic bladder, urinary tract infection, or bladder stones, which could affect voiding, were excluded. To minimize the effect of prostate size on voiding symptoms, patients with a prostate size of over 40 ml were excluded. We used SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis, and multiple linear regression analysis and Pearson's correlation were used to analyze the relationship of the factors. p-values of <0.05 were considered statistically significant.
The patients' mean age was 62.0±9.3 years. Their mean prostate volume was 29.0±5.5 ml (range, 20 to 40 ml), and the median PUA and IPP were 34° (range, 12 to 52°) and 1.7 mm (range, 0 to 5.3 mm), respectively. The mean IPSS, IPSS-ss, and IPSS-vs were 19.0±8.2, 7.3±4.0, and 11.6±5.5, respectively.
The prostate volume had no statistically significant correlation with IPSS, IPSS-ss, or IPSS-vs. Age and IPP were significantly correlated with IPSS (p<0.001), IPSS-ss (p<0.001), and IPSS-vs (p<0.001). PUA had no statistically significant correlation with IPSS or IPSS-ss. However, PUA was significantly correlated with IPSS-vs (p=0.047) (Table 1).
According to the results of the multiple linear regression analysis, IPSS-total, IPSS-ss, and IPSS-vs were significantly associated with age. Whereas IPP was significantly associated with IPSS-total and IPSS-ss, PUA was significantly associated with IPSS-vs (Table 2).
Comparing a higher PUA (≥34°) with a lower PUA (<34°), patients with a higher PUA had a higher IPSS (p=0.001) and a higher IPSS-vs (p=0.001). There was no significant difference in IPP, IPSS-ss, prostate volume, or PSA between the two groups (Table 3).
It is known that the prevalence of histopathologic BPH is age dependent [5]. Usually, histopathologic BPH begins to develop after the age of 40 years, and the prevalence becomes greater than 50% by the age of 60 years and as high as 90% by the age of 85 years. Despite its high prevalence, however, the pathogenesis of BPH is still poorly understood.
BPH is bothersome to patients when it is combined with LUTS. Urodynamic study has been helpful for assessing men with bothersome LUTS suggestive of BPH. However, its invasive nature and potential complications have limited routine clinical application [6,7]. Several recent studies have reported the importance of anatomical factors in evaluating LUTS suggestive of BPH. TRUS is a relatively noninvasive study that could be used on an outpatient clinic basis to evaluate the anatomical structure of the prostate. One of the anatomical factors in BPH evaluation is IPP.
IPP is a morphological change by which the prostate protrudes into the bladder during the process of prostatic enlargement. It is induced by the enlargement of the lateral and median lobes, which leads to the ball-valve type of bladder outlet obstruction (BOO) and abnormal movement of the bladder as a result of inhibition of the funnel effect of the bladder neck at urination [8,9]. Chia et al. [10] described that strong bladder contraction opens the channel between the lateral lobes but accelerates the ball-valve effect by IPP, which induces more BOO than does the enlargement of the lateral lobe or the prostatic enlargement alone.
Whereas IPP is caused by the protrusion of the middle lobe components into the bladder neck, an increased PUA may be the result of a higher bladder neck in men who have no lateral or median prostatic lobe enlargement [3]. Although some urologists suspect that the higher bladder neck might be a causal factor of BPH, the clinical significance of PUA is not well understood.
The prostatic urethra runs through the prostate from the base to the apex, making an anterior angulation of 35° at the proximal part of the verumontanum. This bend divides the urethra into the proximal and distal portions [11].
Cho et al. [3] demonstrated that PUA is inversely associated with maximal flow rate (Qmax). In the study by Cho et al. [3], patients were healthy men (aged 50 to 59 years, prostate volume 30 ml, and Qmax 15 ml/s) who had no evidence of BOO. By applying fluid dynamics to the process of urination in the prostatic urethra, Cho et al. [3] suggested that energy loss in the bending tube in the prostatic urethra could occur during micturition. They insisted that because the increased energy loss resulting from PUA leads to a decrease in urine velocity, the urine flow rate is inversely associated with PUA [2]. Ku et al. [12] found that PUA is correlated with the BOO index. As PUA increases, the BOO index increases. Patients with PUA ≥ 35° were more likely to have equivocal or outlet obstruction than were those with PUA < 35°.
There have been several reports about the relationship between the IPSS and IPP [13-15]. However, despite the published study regarding the relationship between PUA and urinary flow rates, there are few reports about the relationship between the IPSS and PUA.
The IPSS was developed by the WHO and has been widely adopted as a reliable method of detecting and categorizing the severity of LUTS. The IPSS consists of seven items, each of which is rated from 0 (not at all) to 5 (almost all the time). The total score is the sum of the seven items and therefore has a range of from 0 to 35. The greater the score, the more severe the symptoms. The IPSS categorizes patients into three symptom groups: mild, 0-7; moderate, 8-18; and severe, >18.
In the present study, IPP had a statistically significant correlation with the IPSS (p<0.001), IPSS-ss (p<0.001), and IPSS-vs (p<0.001). We found that PUA correlated with only the IPSS-vs (p=0.047) in patients with LUTS or BPH. Multivariate linear regression analysis also showed a statistically significant relation between PUA and the IPSS-vs (p=0.001). We found no correlations between PUA and the IPSS or IPSS-ss. When we compared patients with higher a PUA with patients with a lower PUA, patients with a higher PUA were more likely to have a higher voiding symptom score (p=0.001). This agrees with the concept of PUA being a cause of BOO. Further study to investigate the relationship between PUA and LUTS or BPH may be needed.
Figures and Tables
FIG. 1
(A) Prostatic urethral angle measured by TRUS shows 56°. (B) Intravesical prostatic protrusion measured by TRUS shows 53 mm.
References
1. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003. 170(2 Pt 1):530–547.
2. Cho KS, Kim J, Choi YD, Kim JH, Hong SJ. The overlooked cause of benign prostatic hyperplasia: prostatic urethral angulation. Med Hypotheses. 2008. 70:532–535.
3. Cho KS, Kim JH, Kim DJ, Choi YD, Kim JH, Hong SJ. Relationship between prostatic urethral angle and urinary flow rate: its implication in benign prostatic hyperplasia pathogenesis. Urology. 2008. 71:858–862.
4. Nose H, Foo KT, Lim KB, Yokoyama T, Ozawa H, Kumon H. Accuracy of two noninvasive methods of diagnosing bladder outlet obstruction using ultrasonography: intravesical prostatic protrusion and velocity-flow video urodynamics. Urology. 2005. 65:493–497.
5. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984. 132:474–479.
6. Klingler HC, Madersbacher S, Djavan B, Schatzl G, Marberger M, Schmidbauer CP. Morbidity of the evaluation of the lower urinary tract with transurethral multichannel pressure-flow studies. J Urol. 1998. 159:191–194.
7. Onur R, Ozden M, Orhan I, Kalkan A, Semercioz A. Incidence of bacteraemia after urodynamic study. J Hosp Infect. 2004. 57:241–244.
8. Kuo HC. Clinical prostate score for diagnosis of bladder outlet obstruction by prostate measurements and uroflowmetry. Urology. 1999. 54:90–96.
9. Keqin Z, Zhishun X, Jing Z, Haixin W, Dongqing Z, Benkang S. Clinical significance of intravesical prostatic protrusion in patients with benign prostatic enlargement. Urology. 2007. 70:1096–1099.
10. Chia SJ, Heng CT, Chan SP, Foo KT. Correlation of intravesical prostatic protrusion with bladder outlet obstruction. BJU Int. 2003. 91:371–374.
11. McNeal JE. The prostate and prostatic urethra: a morphologic synthesis. J Urol. 1972. 107:1008–1016.
12. Ku JH, Ko DW, Cho JY, Oh SJ. Correlation between prostatic urethral angle and bladder outlet obstruction index in patients with lower urinary tract symptoms. Urology. 2010. 75:1467–1471.
13. Lee JM, Chung H, Kim TW, Kim HS, Wang JH, Yang SK. The correlation of intravesical prostatic protrusion with storage symptoms, as measured by transrectal ultrasound. Korean J Urol. 2008. 49:145–149.
14. Kim BH, Sohn JC, Park CH, Kim CI. The usefulness of intravesical prostatic protrusion and bladder wall thickness measurement using transabdominal ultrasound in patients with benign prostatic hyperplasia. Korean J Urol. 2005. 46:1180–1185.
15. Kim KH, Kim YS. Correlation of male overactive bladder with intravesical prostatic protrusion. Korean J Urol. 2010. 51:843–846.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download