Abstract
Purpose
We aimed to determine whether a preoperative urodynamic parameter is a valuable predictor for the persistence of OAB symptoms after the AVP repair.
Materials and Methods
65 OAB patients with concomitant POP-Q stage III, IV anterior vaginal wall prolapse underwent a surgical repair were involved. All the patients were subjected to a preoperative urodynamic study, for whom the OABSS on questionnaire were preoperatively recorded. We firstly analyzed the correlation between the BOOI and the OABSS, then randomly divided patients into two groups: the group A (high PdetQmax, BOOI≥20) and the group B (low PdetQmax, BOOI<20). In each group, the OABSS was repeatedly measured post-operatively and the change were analyzed.
Results
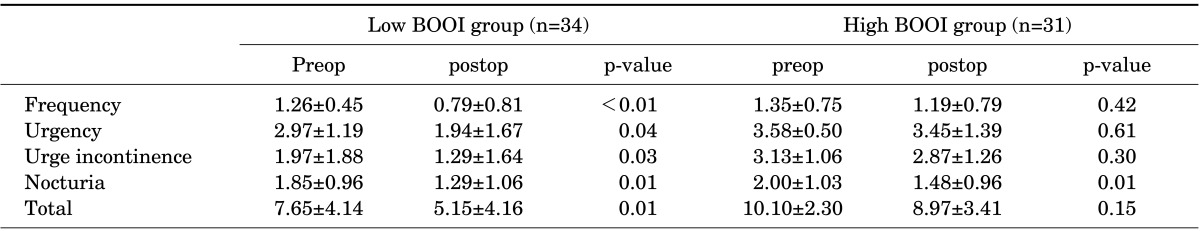
31 patients were classified as the group A and 34 patients were classified as the group B. The group B showed significant decrease of symptom score in daytime frequency (p<0.01), urgency (p=0.04), urge incontinence (p=0.03), nocturnal frequency (p=0.01) and total score (p=0.01). The group A showed no significant decrease of symptom score in daytime frequency (p=0.42), urgency (p=0.61), urge incontinence (p=0.3), total score (p=0.15) except nocturnal frequency (p=0.01).
Conclusions
A preoperative pressure-flow study can be a valuable tool in predicting the OAB symptoms change after the combined AVP repair. While the AVP repair leads to the improvement of OAB symptoms generally, some patients with a higher preoperative PdetQmax are still in need of the additional medical treatment.
Pelvic organ prolapse (POP) is often accompanied by the overactive bladder (OAB) symptoms including urinary frequency, urgency andincontinence. Community-based studies showed a higher prevalence of OAB symptoms in women with POP than those without POP [1-3]. POP is likely to be a causative factor in cases of OAB, which has been proved by many hospital-based studies. Anterior vaginal wall prolapse (AVP) repair by the standard anterior colporrhaphy or using a polypropylene mesh both have reported a very good resolution of OAB symptoms [4-6]. In addition, even the POP repair by a vaginal pessary placement also showed a significant improvement in urgency and urge incontinence [7-9]. While there seems to be a relationship between the OAB symptom resolution and the anatomic repair, however, a subset of patients still complain OAB symptoms after the AVP repair and they are in need of the additional medical treatment.
Despite growing clinical evidence, the pathophysiological mechanisms underlying OAB symptoms in conjunction with POP are not well characterized yet. It is difficult to predict the disease course of OAB after AVP repair. This is because it is not clear which factors can predict whether the OAB symptoms disappear or not after the AVP surgery. To date, few studies clarified this issue but recent report suggested that the persistent OAB symptoms after AVP repair were not related to the demographic factors rather than the preoperative higher PdetQmax [10].
The purpose of this study was to determine whether preoperative urodynamic parameter is a valuable predictor for the persistence of OAB symptoms after AVP repair or not.
Our study prospectively analyzed the change of OAB symptom after a combined prolapse repair. From October 2008 to January 2012, a total of 65 OAB patients with concomitant POP-Q stage III and IV anterior vaginal wall prolapse (AVP) underwent a surgical repair and were enrolled in this study. AVP repair consisted of either a standard anterior colporrhaphy or the use of polypropylene mesh with Gynemesh PS or Prolift system (Ethicon Women's Health and Urology, Somerville, USA). Patients with concomitant apical suspension or posterior repair were included but those with POP repair or incontinence surgery within 1 year prior to baseline evaluation, a concomitant procedure forstress urinary incontinence, anticholinergic medication, neurological disease, urinary tract infection, bladdertumor or stones, or bladder outlet obstruction (BOO) from anetiology other than POP were excluded in order to eliminate possible role as a confounding variable. All patients were subjected to a preoperative urodynamic study, for whom the OAB symptom scores (OABSS) on questionnaire were preoperatively recorded. Urodynamic studies were performed according to the guidelines established by the International Continence Society with an unreduced prolapse state. During cystometry, presence of detrusor overactivity (DO) which was defined as any involuntary rise in detrusor pressure of greater than 5 cm of water during filling associated with urgency or bladderfullness were identified. Besides, the voiding pressure at maximal flow (PdetQmax) and maximal flow rate (Qmax) on a pressure flow study were recorded. This was followed by the calculation of the bladder outlet obstruction index (BOOI). The OABSS represents the sum score of four symptoms such as daytime frequency, nighttime frequency, urgency and urge incontinence, and it has recently been developed and validated. It has been used to estimate the severity of OAB symptoms [11]. The diagnostic criteria is defined as cases in which total scores were higher than 3 points and urgency ones were higher than 2 points. We firstly analyzed the correlation between the bladder outlet obstruction index (BOOI) and the OABSS to investigate the role of obstruction in aggravating the OAB symptoms. Then, we randomly divided patients into two group: the group A (high PdetQmax, BOOI≥20) and the group B (low PdetQmax, BOOI<20). In each group, the OABSS was repeatedly measured after a sufficient length of the post-operative follow-up period of more than 6 months. This was followed by a statistical analysis ofthe changes in the OABSS.
To evaluate the correlation between the BOOI and the OABSS, Pearson correlation coefficients were determined. Besides, a paired t-test was applied to analyze changes in the OABSS between preoperatively and postoperatively. Statistical analysis was done using SPSS ver.13.0 (SPSS Inc., Chicago, IL, USA) with p-values of less than 0.05 were considered to be statistically significant.
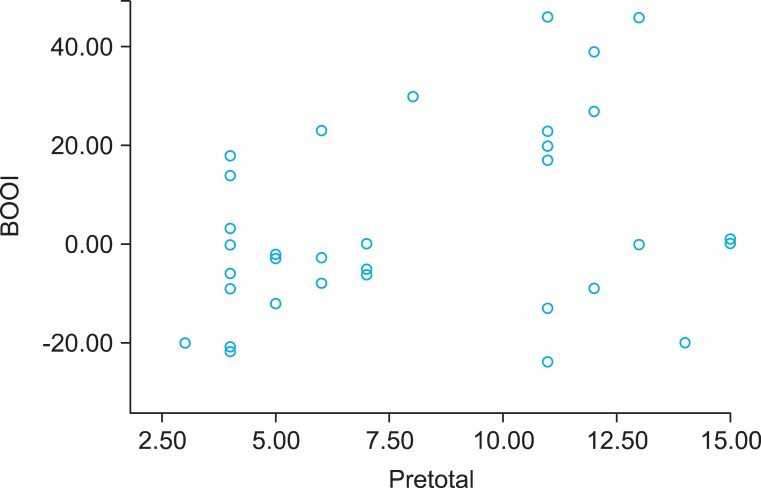
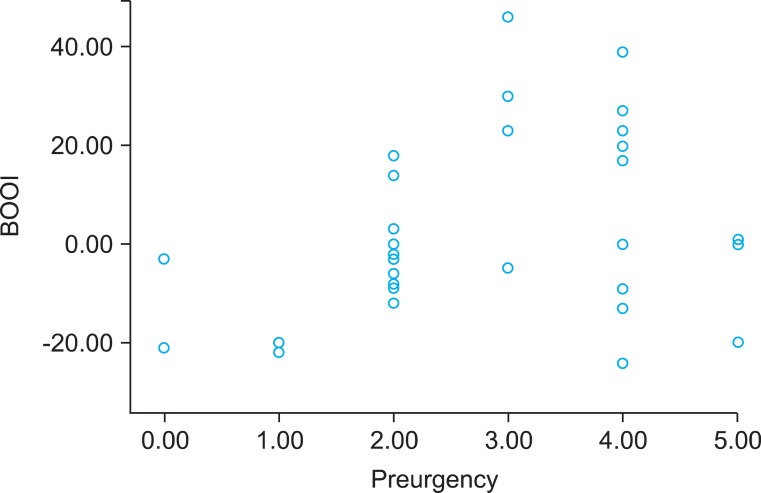
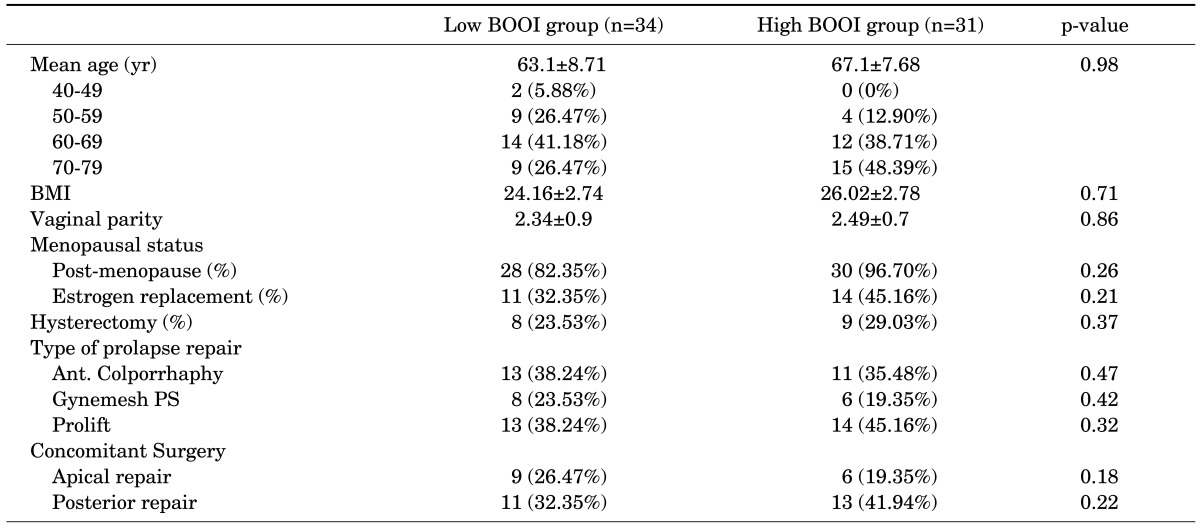
A total of 65 patients (mean age: 64.3±8.2 years) completed a repeated recording of the OABSS after a sufficient length of the postoperative follow-up period (mean value: 24.3±8.3 months). All the patients showed a state of the successful anatomic repair, which was determined based on the absence of recorded failure on physical examination. A correlation analysis showed that the BOOI had a significant positive linear correlation with the total OABSS (r=0.37, p=0.01) and the urgency score (r=0.34, p=0.01) prior to the AVP repair (Figs. 1, 2). According to the calculated BOOI, 31 patients (mean age: 66.1±7.6 years) were classified as the group A (high PdetQmax, BOOI≥20) and 34 patients (mean age: 62.6±8.3 years) were classified as the group B (low PdetQmax, BOOI<20). At baseline, there were no differences in demographic information and concomitant surgeries between the two groups (Table 1). The group B showed significant decrease of symptom score in daytime frequency (p<0.01), urgency (p=0.04), urge incontinence (p=0.03), nocturnal frequency (p=0.01) and total score (p=0.01) after the AVP repair. In the group A, however, there was no significant decrease of symptom score in daytime frequency (p=0.42), urgency (p=0.61), urge incontinence (p=0.3), total score (p=0.15). But there was a significant decrease of nocturnal frequency (p=0.01) (Table 2).
The DO was found in 4 patients (12.9%) of the group A and 3 patients(8.8%) of the group B. All the patients with DO show no improvement of OAB symptoms after the AVP repair.
There are various possible theories regarding the pathophysiology of OAB in relation to POP, which can be summarized in two mechanisms. The most popular and commonly described theory is that BOO induced denervation of autonomic nerve supply to the detrusor muscle and physiologic alteration of detrusor muscle. POP can cause BOO in a large series of cases, which might be a probable mechanism of POP-induced OAB. Various human and animal studies have demonstrated the denervation of autonomic nerve supply to the detrusor muscle in obstructed bladder [12-15]. The reaction to the acetylcholine was super sensitized and reduced nerve-mediated responses werefound compared to the normal stable bladder, which suggests that there is a super sensitivity to neurotransmitters secondarily to partial denervation of the obstructed bladder. Ischemia and hypoxia of the bladder wall caused by bladder distension and contraction might play an important role in this partial denervation in an obstructed bladder [16,17]. BOO also causes a physiologic change in the detrusor muscle. There are a decrease in the cell-to-cell propagation of the electrical activity and a greater instability of the membrane potential, which might cause a depolarization of the cell. Therefore, the detrusor muscle in obstructed bladder is more irritable while synchronous activation is damaged [18]. In addition, it was shown that the BOO model exhibit significant more short latency spinal reflexes than controls while the long latency reflexes were similar in animal study [19]. This suggests that obstruction in rats is accompanied by some degree of neural plasticity resulting in a more prominent spinal reflex that could contribute to the development of the unstable bladder [19-21].
In the present study, we found a strong correlation between the BOOI and the OABSS, which supports the theory that BOO is the mechanism of POP-induced OAB. However, we also experienced the improvement of OAB symptoms after the AVP repair in a fair number of non-obstructive cases. It can therefore be inferred that there might be another probable mechanism of POP-induced OAB.
The second probable theory is that POP induces the occurrence of bladder distension and this might trigger the activation of stretch receptors in the urethelium and then result in the overactivity of detrusor muscle. The stretch receptors in the urothelium release various chemical factors such as ATP, Ach and P2X3 in the condition of bladder distension and this stimulus reaches the sensory neurons and myofibroblasts in the urothelial and suburothelial region which conducts these stimuli to the detrusor muscle [22-24]. Stretching of the bladder wall is likely to happen in anterior vaginal prolapse and this might trigger the activation of stretch receptors resulting indetrusor overactivity. POP generally occur under the damaged state of pelvic ligaments or fascia. If the pelvic ligaments are lax, the vaginal wall cannot be supported by the muscle forces. A lax vaginal wall cannot support the stretch receptors and this may cause gravity to stimulate the nerve ending at the bladder trigone, causing a premature activation of the micturition reflex, expressed as OAB symptoms, such as urinary urgency, frequency, nocturia and urgency incontinence. In the bladder base, the hydrostatic pressure of the urine can not be supported, and bladder stretch receptors may "fire off" to initiate micturition at a lower volume in the same manner. In addition, stretching the bladder base vigorously upwards the pubic symphysis can worsen urgency symptoms [25].
Considering this theory, it is probable that AVP repair may improve OAB symptoms by restoring stretching mechanism in non-obstructive patients. Our results suggest that the AVP with the high BOOI group tends to manifest more severe, irreversible OAB symptoms, whereas that with the low BOOI group does less severe, reversible OAB symptoms. Furthermore, the current study can respond to a question why a subset of patients still complain OAB symptoms even after AVP treatment, whilesome patients experienced an improvement of the symptoms. Fletcher et al. [10] demonstrated a persistent presence of OAB symptoms after the AVP repair had no correlations with the age, parity, BMI or prolapse grade. These authors noted, however, that it was related rather to the pre-operative PdetQmax on their retrospective cohort study. It was suggested that the higher degree of pre-operative PdetQmax appeared as a modest independent risk factor for a persistent urge urinary incontinence after the AVP repair in their study, which is consistent with our results that the degree of BOOI may be a predictor of changes in the OAB symptoms after the AVP repair.
As for a possible source of bias, including a concomitant repair of other compartment prolapse, different baseline characteristics may have an effect on the result. However, there was not significant differences in the baseline demographic factors between the two groups, which can therefore support our result. The presence of preoperative DO also can be a confounding variable. It has been reported the coexisting rate of DO is also higher in advanced POP [26,27].It remains uncertain, however whether the DO arises from POP in coexisting cases as the POP increases the incidence of OAB. According to a recent retrospective study, the urgency and urge urinary incontinence were mostly resolved after the POP surgery in women without DO. In women with DO, however, the urgency and urge urinary incontinence frequently were still present postoperatively [27]. This suggests that urgency without DO is attributed to some temporary POP-related factors such as direct irritation to the prolapsed bladder, in addition to a prolapsed obstruction of the urethra [28]. In our clinical series patients, there were only 4 cases (12.9%) of DO in the high BOOI group and 3 cases (8.8%) in thelow BOOI group and all the patients with DO showed no improvement of OAB symptoms after the AVP repair. This implies that the DO might be an independent finding from the AVP.
One of interesting finding is the improvement of nocturnal frequency in the high BOOI group. As is known, nocturia is a complex disease which is influenced by various factors other than BOO. However, it was recently reported nocturnal bladder capacity (NBC) increased significantly starting from the early postoperative period after resolution of prostatic obstruction [29]. It is probable this phenomenon can occur after resolution of female BOO, which can be a plausible explanation for the improvement of nocturnal frequency in the high BOOI group after the AVP repair. It remains to be clarified by future work.
To our knowledge, the present study is first pilot study to identify the prognostic factors in predicting changes in the OAB symptoms after the AVP repair. Our results showed that a preoperative pressure flow study might be a useful predictive tool in treating OAB patients combined with the AVP. However, our study is limited by the very nature of small sample size. Further larger-scale prospective studies are therefore warranted to draw a definite conclusion.
A preoperative pressure-flow study can be a valuable tool in predicting the OAB symptoms change after the combined AVP repair. While the AVP repair leads to the improvement of OAB symptoms generally, some patients with a higher preoperative PdetQmax are still in need of the additional medical treatments.
References
1. de Boer TA, Cluivers KB, Withagen MI, Milani AL, Vierhout ME. Predictive factors for overactive bladder symptomsafter pelvic organ prolapse surgery. Int Urogynecol J. 2010; 21:1143–1149. PMID: 20419366.
2. Lawrence JM, Lukacz ES, Nager CW, Hsu JW, Luber KM. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet Gynecol. 2008; 111:678–685. PMID: 18310371.

3. Miedel A, Tegerstedt G, Maehle-Schmidt M, Nyrén O, Hammarström M. Symptoms and pelvic support defectsin specific compartments. Obstet Gynecol. 2008; 112:851–858. PMID: 18827128.
4. Digesu GA, Salvatore S, Chaliha C, Athanasious S, Milani R, Khullar V. Do overactive bladder symptoms improve after repair of anterior vaginal wall prolapse? Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:1439–1443. PMID: 17429557.

5. Foster RT Sr, Barber MD, Parasio MF, Walters MD, Wekdner AC, Amundsen CL. A prospective assessment of overactive bladder symptoms in a cohort of elderly women who underwent transvaginal surgery for advanced pelvic organ prolapse. Am J Obstet Gynecol. 2007; 197:82.e1–82.e4. PMID: 17618768.

6. Stanton SL, Hilton P, Norton C, Cardozo L. Clinical and urodynamic effects of anterior colporrhaphy and vaginal hysterectomy for prolapse with and without incontinence. Br J Obstet Gynaecol. 1982; 89:459–463. PMID: 7082603.

7. Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004; 190:1025–1029. PMID: 15118635.

8. Fernando RJ, Thakar R, Sultan AH, Shah SM, Jones PW. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006; 108:93–99. PMID: 16816061.

9. Hanson LA, Schulz JA, Flood CG, Cooley B, Tam F. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006; 17:155–159. PMID: 16044204.

10. Fletcher SG, Haverkorn RM, Yan J, Lee JJ, Zimmern PE, Lemack GE. Demographic and urodynamic factors associated with persistent OAB after anterior compartment prolapse repair. Neurourol Urodyn. 2010; 29:1414–1418. PMID: 20623545.

11. Homma Y, Yoshida M, Seki N, Yokoyama O, Kakizaki H, Gotoh M, et al. Symptom assessment tool for overactive bladder syndrome-overactive bladder symptom score. Urology. 2006; 68:318–323. PMID: 16904444.

12. Gosling JA, Gilpin SA, Dixon JS, Gilpin CJ. Decrease in the autonomic innervations of human detrusor muscle in outflow obstruction. J Urol. 1986; 136:501–504. PMID: 3735523.
13. Harrison SC, Hunnam GR, Farman P, Ferguson DR, Doyle PT. Bladder instability and denervation in patients with bladder outflow obstruction. Br J Urol. 1987; 60:519–522. PMID: 3427336.

14. Harrison SC, Ferguson DR, Doyle PT. Effect of bladder outflow obstruction on the innervation of the rabbit urinary bladder. Br J Urol. 1990; 66:372–379. PMID: 1699626.

15. Sibley GN. The physiological response of the detrusor muscle to experimental bladder outflow obstruction in the pig. Br J Urol. 1987; 60:332–336. PMID: 3690205.

16. Azadzoi KM, Pontari M, Vlachiotis J, Siroky MB. Canine bladder blood flow andoxygenation: changes induced by filling, contraction and outlet obstruction. J Urol. 1996; 155:1459–1465. PMID: 8632611.
17. de Boer TA, Salvatore S, Cardozo L, Chapple C, Kelleher C, van Kerrebroeck P, et al. Pelvic organ prolapse and overactive bladder. Neurourol Urodyn. 2010; 29:30–39. PMID: 20025017.

18. Seki N, Karim OM, Mostwin JL. The effect of experimental urethral obstruction and its reversal on changes in passive electrical properties of detrusor muscle. J Urol. 1992; 148:1957–1961. PMID: 1331551.

19. Steers WD, de Groat WC. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J Urol. 1988; 140:864–871. PMID: 3418824.

20. Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest. 1991; 88:1709–1715. PMID: 1939656.

21. Steers WD, Ciambotti J, Etzel B, Erdman S, de Groat WC. Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol. 1991; 310:401–410. PMID: 1723990.

22. Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes-a possible sensory mechanism? J Physiol. 1997; 505:503–511. PMID: 9423189.
23. Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol. 2004; 171:448–452. PMID: 14665953.
24. Yoshida M, Inadome A, Maeda Y, Satoji Y, Masunaga K, Sugiyama Y, et al. Non-neuronal cholinergic system in human bladder urothelium. Urology. 2006; 67:425–430. PMID: 16461116.

25. Yuan Z, Shen H. Pelvic organ prolapse quantification in women referred with overactive bladder. Int Urogynecol J. 2010; 21:1365–1369. PMID: 20577715.

26. Klutke JJ, Ramos S. Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol. 2000; 182:1378–1381. PMID: 10871452.

27. Araki I, Haneda Y, Mikami Y, Takeda M. Incontinence and detrusor dysfunction associated with pelvic organ prolapse: clinical value of preoperative urodynamic evaluation. Int Urogynecol J Pelvic Floor Dysfunct. 2009; 20:1301–1306. PMID: 19597715.

28. Nguyen JK, Bhatia NN. Resolution of motor urge incontinence after surgical repair of pelvic organ prolapse. J Urol. 2001; 166:2263–2266. PMID: 11696748.

29. Lee CJ, Cho MC, Ku JH, Kim SW, Paick JS. Changes in nocturia after photoselective vaporization ofthe prostate for patients with benign prostatic hyperplasia. Korean J Urol. 2010; 51:531–536. PMID: 20733958.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download