Abstract
Purpose
To determine whether the distance from skin to stone, as measured by computed tomography (CT) scans, could affect the stone-free rate achieved via extracorporeal shock wave lithotripsy (ESWL) in renal stone patients.
Materials and Methods
We retrospectively reviewed the records 573 patients who had undergone ESWL at our institution between January 2006 and January 2010 for urinary stones sized from about 5 mm to 20 mm and who had no evidence of stone movement. We excluded patients with ureteral catheters and percutaneous nephrostomy patients; ultimately, only 43 patients fulfilled our inclusion criteria. We classified the success group as those patients whose stones had disappeared on a CT scan or simple X-ray within 6 weeks after ESWL and the failure group as those patients in whom residual stone fragments remained on a CT scan or simple X-ray after 6 weeks. We analyzed the differences between the two groups in age, sex, size of stone, skin-to-stone distance (SSD), stone location, density (Hounsfield unit: HU), voltage (kV), and the number of shocks delivered.
Results
The success group included 33 patients and the failure group included 10. In the univariate and multivariate analysis, age, sex, size of stone, stone location, HU, kV and the number of shocks delivered did not differ significantly between the two groups. Only SSD was a factor influencing success: the success group clearly had a shorter SSD (78.25±12.15 mm) than did the failure group (92.03±14.51 mm). The results of the multivariate logistic regression analysis showed SSD to be the only significant independent predictor of the ESWL stone-free rate.
Recently, extracorporeal shock wave lithotripsy (ESWL) has become the preferential treatment modality for patients suffering from stones. In the specific case of renal stones [1], ESWL is considered the noninvasive treatment of choice. Although many other such treatment options exist, ESWL is currently regarded as the most effective, especially when applied to stones sized less than 20 mm, irrespective of location [2].
Ever since the advent of noncontrast computed tomography (NCCT), the procedure has become the preferred modality for the diagnosis and treatment of renal calculi, owing primarily to its rapidity and high accuracy relative to previous intravenous pyelography (IVP) methods [3,4]. NCCT provides valuable information confirmative of anatomic abnormalities, in addition to the basic information regarding stone location, size, shape, density, and skin-to-stone distance (SSD). Lately, several experiments assessing the connection between information from the medical field and the efficacy of ESWL have been conducted, and Hounsfield unit (HU), SSD, and stone size have all been identified as key factors in connection with the success of ESWL [5-8]. In the present study, we attempted to determine and describe the most important clinical information collected via NCCT regarding the ESWL stone-free rate.
We retrospectively reviewed the records of 573 patients with urinary stones who had undergone the ESWL procedure between January 2006 and January 2010 at our institution. Inclusion criteria were as follows: 5 to 20 mm, single, radiopaque calculi located within the kidney via NCCT within 1 month of treatment and no evidence of stone migration. We excluded any patients with ureteral catheters as well as all percutaneous nephrostomy patients; ultimately, only 43 patients of the 573 records reviewed met the inclusion criteria.
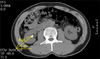
All patients were treated by using the same electroconductive lithotriptor (Sonolith Praktis; EDAP TMS, Vaulx-en-Velin, France) under fluoroscopy. The shockwave was fixed at twice per second and the number of shockwaves was adjusted from 2,500 to 3,000; the launch intensity was conducted within 14.5 to 15.5, determined by the pain reported by the patients while ESWL was being carried out. The longest stone size by measurement on NCCT was used, and we measured stone density (HU) and location by using NCCT images. For SSD measurement, we took a measurement from the point of the largest stone diameter at a 45° angle from the horizontal (Fig. 1). We used the Marosis view during the measurement procedure.
We included in the success group all cases in which the stone disappeared on the CT scan or simple X-ray within 6 weeks after ESWL and in the failure group all patients in whom clinically significant residual stone fragments remained on a CT scan or simple X-ray after 6 weeks. We analyzed the differences between the two groups in age, sex, size of stone, SSD, stone location, density (HU), voltage (kV), and the number of shocks delivered. All statistical analyses were performed by using the Student's t-test, Pearson's chi-square test for univariate analysis, and logistic regression for multivariate analysis. SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used, and p-values less than 0.05 were considered statistically significant.
The mean age of the patients was 55.7±13.76 years. The proportion of males to females was 1.15:1 (23 males and 20 females). Among 43 patients, 33 (69.7%) were included in the success group and 10 patients (30.3%) in the failure group in terms of ESWL efficacy. All patients had one solitary stone, with a mean size of 11.09±4.23 mm. In 28 patients, the stone was more than 10 mm in size (by diameter). The average stone size in the success group was 10.55±4.73 mm and the average stone size in the failure group was 12.90±3.67 mm (p=0.150). With regard to HU, the success group had values of 784.12±306.35 and the failure group of 837.50±391.45. The differences between the groups were not statistically significant. With regard to stone locations in all patients, the results were as follows: lower pole (19 patients), renal pelvis (13 patients), central region (8 patients), and upper pole (3 patients).When ESWL voltage and number of shocks were compared between the two groups, the results were as follows: 15.13±0.60 kV and 15.42±0.25 kV, respectively (p=0.148), and 2,939.39±272.64 and 3,100.00±216.22, respectively (p=0.123) Only SSD differed to a statistically significant degree when compared between the two groups: the mean SSD was 78.25±12.15 mm in the success group and 92.03±14.51 mm in the failure group (p<0.05) (Table 1).
Only SSD was a factor influencing success in the multivariate logistic regression analysis. Sex, stone size, HU, and kV all were identified as factors extraneous to success (Table 2).
Currently in Korea, the use of NCCT has become increasingly common owing to its accuracy and convenience, although it should be noted that IVP techniques remain in broad use for the diagnosis of urinary calculi [9-11]. Although IVP has conventionally been extensively used as an effective diagnostic method [12], it is limited in terms of factors such as contrast side effects, preparation, NPO time, and total test time [13,14]. IVP images can be disturbed by bony structures and bowel gas. Additionally, IVP can derive only a limited amount of information from a radiolucent stone. NCCT has been used previously as the first-line technique for stone evaluation, and this procedure is becoming common in the research field. NCCT is one of the most accurate diagnostic methods [11]. Also, NCCT not only provides information about the stone itself, but also plenty of information about abnormalities of the organs and urinary tract (e.g., renal mass, duplicated ureter, bladder mass, gallbladder stone, pancreatitis, appendicitis, diverticulitis of large intestine) [15].
Recently, NCCT has been evaluated for use not only for the diagnosis of stones but also for the prediction of ESWL treatment results, which is analyzed by use of various metrics. HU is simply a charting of stone density. Some studies have reported that the HU value is inversely related to the ESWL success rate [4,7,16,17]. Another study noted no noticeable differences in HU between a success group and a failure group. Indeed, according to preliminary research, there are no statistically significant differences in HU between an ESWL success group and a failure group [8,18]. This result may indicate some measurement biases deriving from the lack of a standardized measurement method, especially in light of different HU values observed after the location of measurement and ROI on the same stones. In addition to HU, ESWL, which is predictive of favorable treatment results, appears to be a vigorous indicator of body mass index and stone size. However, the results of each of these previous studies differ slightly from one another. In this study, we found no significant correlation between stone size and the stone-free rate. However, stone size has been reported in other studies as a significant predictor of the results of ESWL [17,19,20]. This is because of different stone characteristics such as stone density and location.
Electrohydraulic lithotriptors, which generate a shock wave beginning at an electrode and moving to a second point (level of stone), result in a longer migration length for larger patients. Therefore, the success rate could be reduced as a result of a drop in the stone fragmentation efficacy. According to a study conducted by Pareek et al. [6] the SSD of the stone-free group was markedly lower than the SSD of the residual stone group (p<0.01). Furthermore, the efficacy of ESWL drops substantially when SSD exceeds 10 cm. Another study demonstrated a remarkable difference in SSD between the success group and the failure group; our study found the same result. According to the research of Weld et al. [19] SSD is the only factor predictive of treatment efficacy on pelvic/ureteropelvic junction stones. In our study, ureter stones, which are affected by bowel gas and stone location, were ruled out. Additionally, we analyzed renal stones by their location and determined that SSD was the sole factor predictive of treatment efficacy.
SSD can be readily measured by NCCT and is correlated with the success of ESWL. The prediction of ESWL success by measurement of SSD improves the efficacy of the remedial value of ESWL and also demonstrates the practicality and efficacy of NCCT for the diagnosis of urinary stone patients.
Figures and Tables
FIG. 1
The measurement of skin-to-stone distance at 45° on an axial scan of non-contrast computed tomography. SSD: skin to stone distance.
References
1. Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980. 2(8207):1265–1268.
2. Wilson WT, Preminger GM. Extracorporeal shock wave lithotripsy. An update. Urol Clin North Am. 1990. 17:231–242.
3. Yilmaz S, Sindel T, Arslan G, Ozkaynak C, Karaali K, Kabaalioğlu A, et al. Renal colic: comparison of spiral CT, US and IVU in the detection of ureteral calculi. Eur Radiol. 1998. 8:212–217.
4. Saw KC, McAteer JA, Fineberg NS, Monga AG, Chua GT, Lingeman JE, et al. Calcium stone fragility is predicted by helical CT attenuation values. J Endourol. 2000. 14:471–474.
5. Pareek G, Armenakas NA, Fracchia JA. Hounsfield units on computerized tomography predict stone-free rates after extracorporeal shock wave lithotripsy. J Urol. 2003. 169:1679–1681.
6. Pareek G, Hedican SP, Lee FT Jr, Nakada SY. Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology. 2005. 66:941–944.
7. Joseph P, Mandal AK, Singh SK, Mandal P, Sankhwar SN, Sharma SK. Computerized tomography attenuation value of renal calculus: can it predict successful fragmentation of the calculus by extracorporeal shock wave lithotripsy? A preliminary study. J Urol. 2002. 167:1968–1971.
8. El-Nahas AR, El-Assmy AM, Mansour O, Sheir KZ. A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: the value of high-resolution noncontrast computed tomography. Eur Urol. 2007. 51:1688–1693.
9. Katz DS, Lane MJ, Sommer FG. Non-contrast spiral CT for patients with suspected renal colic. Eur Radiol. 1997. 7:680–685.
10. Arac M, Celik H, Oner AY, Gultekin S, Gumus T, Kosar S. Distinguishing pelvic phleboliths from distal ureteral calculi: thin-slice CT findings. Eur Radiol. 2005. 15:65–70.
11. Dalla Palma L, Pozzi-Mucelli R, Stacul F. Present-day imaging of patients with renal colic. Eur Radiol. 2001. 11:4–17.
12. Osborne ED, Sutherland CG, Scholl AJ Jr, Rowntree LG. Roentgenography of urinary tract during excretion of sodium iodine. JAMA. 1983. 250:2848–2853.
13. Fielding JR, Steele G, Fox LA, Heller H, Loughlin KR. Spiral computerized tomography in the evaluation of acute flank pain: a replacement for excretory urography. J Urol. 1997. 157:2071–2073.
14. Kim HS, Jang SW, Jeong YB, Kim YG, Kim JS. The usefulness of unenhanced helical computerized tomography in patients with urinary calculi. Korean J Urol. 2003. 44:796–800.
15. Ahn SS, Lee SH, Kang IM. The value of non-enhanced spiral CT in the diagnosis of suspected urolithiasis. Korean J Urol. 2002. 43:1008–1013.
16. Perks AE, Schuler TD, Lee J, Ghiculete D, Chung DG, D'A Honey RJ, et al. Stone attenuation and skin-to-stone distance on computed tomography predicts for stone fragmentation by shock wave lithotripsy. Urology. 2008. 72:765–769.
17. Park YI, Yu JH, Sung LH, Noh CH, Chung JY. Evaluation of possible predictive variables for the outcome of shock wave lithotripsy of renal stones. Korean J Urol. 2010. 51:713–718.
18. Kim JH, Moon YT. Predicting the therapeutic effect of extracorporeal shockwave lithotripsy by non-enhanced computed tomography in renal stones. Korean J Urol. 2008. 49:252–256.
19. Weld KJ, Montiglio C, Morris MS, Bush AC, Cespedes RD. Shock wave lithotripsy success for renal stones based on patient and stone computed tomography characteristics. Urology. 2007. 70:1043–1046.
20. Wang LJ, Wong YC, Chuang CK, Chu SH, Chen CS, See LC, et al. Predictions of outcomes of renal stones after extracorporeal shock wave lithotripsy from stone characteristics determined by unenhanced helical computed tomography: a multivariate analysis. Eur Radiol. 2005. 15:2238–2243.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download