Abstract
Mucosal spreading of urothelial tumors to the seminal vesicles is very rare. We experienced a case of mucosal involvement of the seminal vesicles by a bladder tumor in a 72-year-old man. The patient had a history of transurethral resection for invasive urothelial carcinoma of the bladder 8 years previously. Radical cystoprostatectomy was performed owing to recurrent and multiple urothelial carcinoma in situ. Microscopically, the urothelial carcinoma in situ was throughout the mucosa of the urinary bladder, both ureters, the prostate, and the left seminal vesicle. To date, the implication of mucosal involvement of the seminal vesicles by urothelial carcinoma is unclear. However, careful microscopic examination is needed to avoid an erroneous diagnosis.
Involvement of the seminal vesicles by primary urothelial carcinoma of the urinary bladder is uncommon [1,2]. Involvement of the seminal vesicles is demonstrated in two different patterns, which consist of direct extension through the perivesical fat and mucosal spread [3]. Most cases show direct extension, whereas mucosal spread occurs in only a minority of cases [1-3]. Of these, the direct invasion of the seminal vesicle is classified as pT4 according to the current tumor-node-metastasis (TNM) staging system [1,4]. However, the clinical significance of mucosal spread to the seminal vesicles remains unclear owing to its infrequency. Therefore, it is important to recognize not only the involvement of the seminal vesicles by urothelial carcinoma of the bladder but also the pattern of involvement, that is, whether direct invasion or mucosal spread is present. Herein, we report a case of primary urothelial carcinoma in situ of the bladder with mucosal spread to the seminal vesicles. To our knowledge, this is the first such report in Korea.
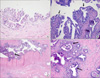
A 75-year-old man was referred to Chungbuk National University Hospital owing to urinary frequency. He had a history of transurethral resection of the bladder for invasive urothelial carcinoma 8 years previously. The tumor was of a low grade and invaded the subepithelial connective tissue. However, proper muscle involvement was not evaluable because the specimen had no proper muscle component. The patient had been treated with Bacillus Calmette-Guerin (BCG) and had lived without recurrence. The patient underwent a transurethral biopsy under the clinical impression of recurrent urothelial cancer. There was widespread urothelial carcinoma in situ; therefore, radical cystoprostatectomy with orthotopic bladder substitution (Ghoneim) was carried out. Grossly, the mucosal surface of the urinary bladder showed multifocal flat erythematous lesions, and there were no remarkable lesions in the remainder. The microscopic findings showed multiple urothelial carcinoma in situ lesions throughout the mucosa of the urinary bladder and both ureters (Fig. 1A). The left seminal vesicle was covered by large polygonal tumor cells, which were confined to the mucosa in single to several layers (Fig. 1B). The tumor cells had large and hyperchromatic nuclei with distinct nuclear membranes and relatively abundant eosinophilic cytoplasm. In addition, bizarre-shaped tumor cells showing irregular hyperchromatic or smudged nuclei were occasionally identified. The tumor cells mostly involved the mucosa between the epithelial cells and the basal lamina of the seminal vesicle, a feature that is referred to as pagetoid spread. In some areas, the entire thickness of the mucosa was replaced by tumor cells. The mucosa of the ejaculatory duct and adjacent prostatic acini and duct were also scattered with tumor cells (Fig. 1C and 1D). On the basis of the overall findings, we considered that the urothelial carcinoma in situ of the urinary bladder revealed mucosal spread to the seminal vesicle along the ejaculatory duct. In addition, there was an incidental prostatic adenocarcinoma, which was a small, solitary lesion with a Gleason score of 6. At the time of the surgery, the patient's serum prostate-specific antigen level was 0.87 ng/ml. The patient had no evidence of disease for 5 months after surgery.
Involvement of the seminal vesicles by urothelial carcinoma of the bladder is uncommon [1]. The frequency of seminal vesicle involvement has been reported to be approximately 3% in cystoprostatectomy cases [2-4]. Daneshmand et al. [1] reported that only 8% of pT4 transitional cell carcinoma cases showed invasion to the seminal vesicle. Ro et al. [3] pointed out that insufficient histologic sections from the seminal vesicle may result in underestimation of seminal vesicle involvement.
The involvement of the seminal vesicle is demonstrated in two distinct patterns [1,3]. One is direct invasion through the bladder wall, and the majority of cases fall under this pattern [1-3]. The other pattern is pagetoid mucosal spread, which is uncommon. Multiplicity of the urothelial carcinoma within the bladder frequently occurs. However, multiple mucosal involvements of the urothelial carcinoma into adjacent organs are not common; therefore, the pathogenesis of this phenomenon has not been well explained. Several possibilities have been suggested, including pagetoid mucosal spread, tumor cell implantation, and de novo development of urothelial carcinoma [2,3]. De novo development from the seminal vesicle epithelium seems less plausible, because there is no transition between normal epithelium and the tumor cells [3]. Although the possibility of implantation via sloughing of tumor cells cannot be completely excluded, it also seems unlikely, inasmuch as the tumor cells usually demonstrate the pagetoid feature [2,3]. In this case, widespread urothelial carcinoma in situ was identified in the bladder, both ureters, the ejaculatory duct of the prostate, and the seminal vesicle, and these tumor cells seemed to grow continuously. Therefore, our case supports the notion of pagetoid mucosal extension of urothelial carcinoma rather than de novo development.
In this case, differential diagnosis for metastatic carcinoma, primary seminal vesicle carcinoma, and atypical degenerated epithelial cells of the seminal vesicle was required. First, the possibility of metastasis from adjacent organs, such as the prostate and the rectum, should be considered. In fact, prostatic adenocarcinoma commonly coexists in bladder tumor patients [5]. Immunohistochemical staining for prostate-specific antigen (PSA), cytokeratin (CK)7, and CK20 may be helpful for distinguishing urothelial carcinoma (PSA-, CK7+, and CK20+) from prostatic adenocarcinoma (PSA+) or rectal carcinoma (CK7- and CK20+) [6]. In this case, the tumor cells were negative for PSA and positive for CK7. Thus, this result supported the evidence for seminal vesicle involvement of urothelial carcinoma. Second, primary adenocarcinoma as well as squamous cell carcinoma can occur in the seminal vesicles [6,7]. However, there must be no other primary carcinoma in the body to establish a diagnosis of primary carcinoma of the seminal vesicle. In addition, Ormsby et al. [6] reported that primary adenocarcinoma of the seminal vesicle shows cancer antigen-125 positivity, which is helpful for making a diagnosis. Finally, the seminal vesicle frequently shows atypical epithelial cells associated with aging (so-called "monstrous cells") [8]. Like the tumor cells of this case, the monstrous cells show markedly enlarged nuclei with an irregular shape and hyperchromasia. However, the monstrous cells contain lipofuscin granules and intranuclear inclusions beyond what is demonstrated in urothelial carcinoma or prostatic adenocarcinoma [9].
The question we must ask here is how the involvement of the seminal vesicle is classified in the aspect of staging. Invasion of the seminal vesicles is classified as pT4 according to the current TNM staging system. Because direct invasion to the seminal vesicle portends poor prognosis, this assignment is warranted in the case of direct extension [1,4]. However, the clinical significance of mucosal spread to the seminal vesicles remains unclear. Esrig et al. [10] reported that mucosal spread to the seminal vesicle adversely affects the prognosis of urothelial carcinoma of the bladder unlike involvement of the prostate only. On the other hand, many authors have insisted that mucosal spread should be under a separate subcategory regarding its better prognosis compared with direct invasion [2-4]. Thus, awareness of seminal vesicle invasion is important to verify the clinicopathologic implications.
In summary, we experienced a case of bladder urothelial carcinoma in situ with mucosal spread to the seminal vesicle. It may be difficult to distinguish this from the metastatic carcinoma from other organs and the primary carcinoma and the reactive epithelial atypia of the seminal vesicles. Recognition of the involvement of seminal vesicles by urothelial carcinoma as well as the pattern of this involvement is important for determining the clinicopathologic implications.
Figures and Tables
FIG. 1
(A) Urothelial carcinoma in situ is observed in the urinary bladder, which epithelium is covered by the proliferating large tumor cells with hyperchromatic nuclei (H&E, ×200). (B) The mucosa of the seminal vesicle shows pagetoid spread of the tumor cells. The tumor cells infiltrate between the epithelial cells of the seminal vesicle (arrow) and the basal lamina (H&E, ×200). (C) A focus of the ejaculatory duct (arrow) and (D) the prostatic acini and ducts (arrow) are also involved by tumor cells (C, H&E, ×40; D, H&E, ×100).
ACKNOWLEDGEMENTS
This work was supported by a research grant of the Chungbuk National University in 2010.
References
1. Daneshmand S, Stein JP, Lesser T, Quek ML, Nichols PW, Miranda G, et al. Prognosis of seminal vesicle involvement by transitional cell carcinoma of the bladder. J Urol. 2004. 172:81–84.
2. Montie JE, Wojno K, Klein E, Pearsall C, Levin H. Transitional cell carcinoma in situ of the seminal vesicles: 8 cases with discussion of pathogenesis, and clinical and biological implications. J Urol. 1997. 158:1895–1898.
3. Ro JY, Ayala AG, el-Naggar A, Wishnow KI. Seminal vesicle involvement by in situ and invasive transitional cell carcinoma of the bladder. Am J Surg Pathol. 1987. 11:951–958.
4. Volkmer BG, Kufer R, Maier S, Bartsch G Jr, Bach D, Hautmann R, et al. Outcome in patients with seminal vesicle invasion after radical cystectomy. J Urol. 2003. 169:1299–1302.
5. Revelo MP, Cookson MS, Chang SS, Shook MF, Smith JA Jr, Shappell SB. Incidence and location of prostate and urothelial carcinoma in prostates from cystoprostatectomies: implications for possible apical sparing surgery. J Urol. 2004. 171(2 Pt 1):646–651.
6. Ormsby AH, Haskell R, Jones D, Goldblum JR. Primary seminal vesicle carcinoma: an immunohistochemical analysis of four cases. Mod Pathol. 2000. 13:46–51.
7. Tabata K, Irie A, Ishii D, Yanagisawa N, Iwamura M, Baba S. Primary squamous cell carcinoma of the seminal vesicle. Urology. 2002. 59:445.
8. Habanec B. Monstrous epithelium in the seminal vesicles and epididymis. Cesk Patol. 1984. 20:73–78.
9. Hameed O, Humphrey PA. Pseudoneoplastic mimics of prostate and bladder carcinomas. Arch Pathol Lab Med. 2010. 134:427–443.
10. Esrig D, Freeman JA, Elmajian DA, Stein JP, Chen SC, Groshen S, et al. Transitional cell carcinoma involving the prostate with a proposed staging classification for stromal invasion. J Urol. 1996. 156:1071–1076.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download