Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | FIG. 1Estimation of kidney volume by use of the immersion method. (A) A laboratory jar was filled with distilled water, was placed on the scale, and weighed. (B) The kidney was suspended by a thin thread in the laboratory jar. The kidney did not have to touch the bottom or sides of the jar. The new weight in grams minus the weight of the laboratory jar and water divided by the specific gravity of the distilled water (-1.0) was considered the primary volume of the kidney in cubic centimeters. |
 | FIG. 2Isotropic uniform random sectioning by use of the orientator method. (A) The kidney was cleaned. (B) It was placed on the circle such that each half of it was divided into 10 equal parts. A random number between 0 and 9 was selected. The kidney was sectioned into two parts at the direction of the selected number (here 4). (C) The cut surface of one part of the kidney was, then, placed on the 0-0 direction of the second circle with 10 unequal divisions. The circle division was done according to the cosine of the angels. Then, another random number was selected and the second cut was done (here 1). The parts were sectioned into parallel slabs (D) The cut surface of the other part of the kidney was placed vertically on the second circle. Again, a new number and direction (here 6) was selected and cut. This part was also sectioned into parallel slabs. |
 | FIG. 3Estimation of shrinkage and slide preparation. (A) The entire kidney was sectioned into slabs with a blade and was placed in the direction of the isotropic uniform random cut with an interval of -0.5 mm. Then, the slabs (8 to 12 slabs) were collected. A circle (left arrow) was punched from a kidney slab (right arrow) by a trocar. The diameter of the circular piece and the area of the circle were estimated using the usual formula for calculating the area of a circle. (B) The cut surface of the slabs and the circle were embedded in the paraffin block. (C) Sectioning of the block using the microtome blade (5 µm thicknesses) is demonstrated. After staining with Heidenhain's Azan trichrome, the area of the circular piece was measured again and the volume shrinkage was calculated by using the following formula: volume shrinkage:=1-(AA/AB)1.5 where AA and AB, respectively,represent the area of the circular piece after and before the processing, the sectioning, and the staining. After estimating the shrinkage, the final volume of the kidney (the reference space) was computed by using the following formula: Vfinal:=Vprimary×(1-volume shrinkage). (D) The microscopic fields were sampled in a systematic random design. The fields were sampled and analyzed at equal intervals along the X- and Y-axis by using a stage micrometer. This procedure was continued until all the sections had been studied. |
 | FIG. 4The volume density estimation using the point-counting method. A gird of points was superimposed upon the images of the renal sections were viewed on the monitor. The volume density (fractional volume) (Vv) of the renal cortex, the medulla, the glomeruli, the proximal convoluted tubules, the distal convoluted tubule, the collecting ducts, the Henle's loop, the vessels, and the connective tissue was obtained by using the point-counting method and the following formula: Vv=P(structure)/P(total) where P(structure) and P(total) represent the sum of the number of the points hitting the structure and all sampled fields of the kidney, respectively. The point is the right upper corner of the cross (the arrow) (Heidenhain's AZAN trichrome stain, ×200). |
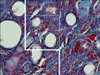 | FIG. 5The length density estimation in a kidney with an obstructed ureter. The counting frame with exclusion lines (bold lines of the left and lower borders) and inclusion lines (thin lines of the right and upper borders) was superimposed on the images. The tubules that were either completely or partly inside the counting frame and did not touch the exclusion lines were counted. The length density (Lv) of each tubule was calculated by using the following formula: Lv:=2×ΣQ/ΣAT where ΣQ denotes the total number of each tubule profiles counted per kidney and AT was the test area that was calculated by multiplying the area of the frame (here was 2,700 µm2) and the total number of the counted frames (Heidenhain's AZAN trichrome stain, ×400). |
 | FIG. 6A comparison of the renal tissue in different groups. (A) The normal tissue. (B) The renal tissue after the unilateral ureteral obstruction (UUO). (C) The renal tissue after the UUO+taxol. (D) The renal tissue after the UUO+taurine (Heidenhain's AZAN trichrome stain, ×200). |
TABLE 1

Values are presented as mean±standard deviation.
a: Sham-operated animals in which laparotomy was done without any procedure and no treatment was given, b: animals underwent a surgery to induce unilateral uretral obstruction, c: animals underwent a surgery to induce unilateral ureteral obstruction and received taxol (0.3 mg/kg twice per week intraperitoneally) for 28 days, d: animals underwent a surgery to induce unilateral ureteral obstruction and received taurine (7.5 mg/kg/d) given by the gavage for 28 days.
TABLE 2

Values are presented as mean±standard deviation.
PCT, proximal convoluted tubule; DCT, distal convoluted tubule.
a: Sham-operated animals in which laparotomy was done without any procedure and no treatment was given, b: animals underwent a surgery to induce unilateral uretral obstruction, c: animals underwent a surgery to induce unilateral ureteral obstruction and received taxol (0.3 mg/kg twice per week intraperitoneally) for 28 days, d: animals underwent a surgery to induce unilateral ureteral obstruction and received taurine (7.5 mg/kg/d) given by gavage for 28 days, e: p<0.01, group 2 vs. (group 3) or (group 4), f: p<0.01, (group 3) vs. (group 4).
TABLE 3

Values are presented as mean±standard deviation.
PCT, proximalconvoluted tubule; DCT, distal convoluted tubule.
a: Sham-operated animals in which laparotomy was done without any procedure and no treatment was given, b: animals underwent a surgery to induce unilateral uretral obstruction, c: animals underwent a surgery to induce unilateral ureteral obstruction and received taxol (0.3 mg/kg twice per week intraperitoneally) for 28 days, d: animals underwent a surgery to induce unilateral ureteral obstruction and received taurine (7.5 mg/kg/d) given by gavage for 28 days, e: p<0.01, group 2 vs. (group 3) or (group 4), f:p<0.01, (group 3) vs. (group 4).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download