Abstract
Wilms' tumor is one of the most frequent malignant neoplasms in childhood. Advances in treatment modalities such as the combination of chemoradiation therapy with surgery have enhanced overall survival. However, recurrence of Wilms' tumor is still a problem. In this case, a 28-year-old female had experienced intermittent abdominal pain, and the computed tomography scan showed a huge pelvic mass. The patient had a history of radical nephrectomy for Wilms' tumor with concurrent chemotherapy at the age of three. The pelvic mass was resected in February 2010 and was confirmed to be a recurrent Wilms' tumor. The recurrent tumor showed a classic triphasic Wilms' tumor growth pattern with frequent mitoses and tumor necrosis. Our case is an extraordinary case of a long-delayed recurrent Wilms' tumor after 25 years, which is the longest disease-free interval ever reported. The possible effects of chemotherapy as well as some other mechanisms of this late relapse are discussed.
Wilms' tumor is one of the most common malignant neoplasms in childhood. It typically occurs in children less than 5 years of age and rarely in adults. Wilms' tumor is highly responsive to chemotherapy and radical nephrectomy, with about 90% of patients surviving at least 5 years, particularly patients with localized disease and favorable histology [1-3]. Despite therapeutic improvements, however, a subset of patients suffers from tumor relapse. In developing countries, the relapse rate has been reported to be 15 to 20% [4,5].
Recently, we experienced a case of Wilms' tumor that recurred 25 years after the primary diagnosis and treatment. To the best of our knowledge, this is the most delayed case of recurrence ever reported in the English literature.
A 28-year-old female had experienced intermittent abdominal pain for 3 months and visited the Asan Medical Center, Seoul, South Korea, for further evaluation and treatment. An abdominal-pelvic computed tomography scan taken in January 2010 at the Asan Medical Center revealed a huge mass involving most of the lower abdomen and pelvic cavity with multiple septations and areas of enhancement (Fig. 1). The provisional diagnosis on the basis of the image findings was either borderline or malignant ovarian neoplasm. Neither ascites nor metastatic lymphadenopathy was found.
The patient had a history of radical nephrectomy for Wilms' tumor of the right kidney 25 years previously, at the age of 3 years, in April 1985 at an outside hospital. After surgery, she had been treated with intravenous injection of dactinomycin 25 mcg for 5 days and vincristine 1 mg on the 7th day after the operation. Since then, she had been healthy with no evidence of recurrence or metastasis until she noticed abdominal pain in September 2009.
Under the clinical diagnosis of an ovarian tumor, the pelvic mass was resected in February 2010. During surgery, the mass was noted to occupy the entire lower abdominal cavity without involving both adnexa. Intraoperative consultation was made and the frozen section diagnosis was malignant gastrointestinal stromal tumor or other malignant mesenchymal tumor such as leiomyosarcoma with pending evaluation of permanent sections.
The original slides of the Wilms' tumor, resected 25 years ago, could not be reviewed because the slides were not available in the file of the outside hospital. However, the outside pathology report stated that the size of the tumor was 12 cm and it was located at the right kidney. The tumor was abutting the head of the pancreas, and in the operation record it was written that the tumor was not completely excised. Microscopically, the tumor was shown to be a classic Wilms' tumor with predominant epithelial tubular elements and minor blastemal and mesenchymal components. No anaplasia or lymphovascular invasion was recorded, and the mitotic count was stated as scant. The final diagnosis of the nephrectomy specimen was Wilms' tumor, stage III.
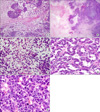
Grossly, the resected tumor in February 2010 was a 30-cm, previously ruptured, solid and cystic mass. On the cut surface, the tumor was heterogeneous, pinkish tan to yellow, and had both solid and friable areas. Multifocal hemorrhagic, cystic, and necrotic changes were also noted (Fig. 2). Microscopically, the tumor was composed of three different cell types, epithelial, mesenchymal, and blastemal components (Fig. 3A). Areas of tumor necrosis were seen (Fig. 3B). The mesenchymal tumor cells were short, spindle-shaped to round cells with scanty cytoplasm in a myxoid background (Fig. 3C). The epithelial cell component formed tumor cell nests with glandular architecture (Fig. 3D). The majority of the tumor compartment was an undifferentiated blastemal cell component. Mitoses were frequently observed up to 30/10 HPFs, particularly in the blastemal component. Hyperchromatism was also observed, but significant pleomorphism was not obvious (Fig. 3E). Immunohistochemical staining was done and the results were as follows: focal positive for cytokeratin in the epithelial component but negative in the blastemal and mesenchymal components (Fig. 4A). The Ki-67 labeling index was positive in about 50% of the tumor cells (Fig. 4B), and p53 staining was positive in about one third of the tumor cells in all three elements. The immunohistochemical staining for WT-1, S-100 protein, SMA, desmin, myogenin, myoglobin, CD117, and CD34 was negative in all three components.
On the basis of the histologic findings in conjunction with the immunohistochemical findings, the tumor was diagnosed as a recurrent classic Wilms' tumor without anaplastic changes.
Wilms' tumor relapse is not uncommon and accounts for approximately 15% of Wilms' tumors with favorable histology [6]. Most recurrent Wilms' tumors have been reported to occur in the first 2 years after the primary diagnosis. By contrast, long-delayed late recurrences, defined as 5 years after the initial diagnosis, are extremely rare events, and most of them have been reported in the form of individual case reports (Table 1) [1,7-10].
Among the previously reported late recurrence cases, all but two cases showed similar histological features between the original and the recurrent tumors, with persistence of immature blastematous elements or nephrogenic rests [1,7]. The two cases with different morphologic features between the primary and recurrent tumors had a tumor relapsing interval of 23 years. They both showed differentiated epithelial cell components and extensive mature skeletal muscle elements, which are known to be resistant to chemotherapy. In the present case, the recurrent tumor showed histologic features similar to the original tumor, with the classic Wilms' tumor histology, according to the pathology report, although there were some proportional differences.
Preoperative chemotherapy is thought to be associated with changes in histological features, and the therapeutic effects can vary according to the different tumor components of a Wilms' tumor. In a recent report by Senetta et al. [1] preoperative chemotherapy was shown to be able to enhance tumor differentiation, thus inducing tumor maturation with ablation of immature tumor components. In that study, the authors hypothesized that there are two different types of Wilms' tumor recurrence as follows: 1) the first group comprises cases in which chemotherapy is not effective, in which tumor recurrences have a short disease-free interval with a more undifferentiated tumor and more aggressive behavior, and 2) the second group comprises cases in which chemotherapy is effective, and tumor recurrences show a long disease-free interval with a high degree of differentiation and better survival related to the previous therapy. It can be postulated that if treatment is effective, the immature components of the tumor are more sensitive to preoperative chemotherapy and radiation treatment and are prone to be ablated more easily than are the mature components [1].
The present case recurred 25 years after the primary Wilms' tumor was diagnosed and treated with combined surgery and chemoradiation, and to the best of our knowledge, this is the longest disease-free interval reported in the English literature. According to the outside hospital pathology report, the primary kidney tumor of the current reported case was mostly composed of epithelial tubular components with lesser amounts of blastemal and mesenchymal components and without anaplastic features. However, the recurrent tumor showed much more blastemal components with pronounced mitoses, although there were no features of anaplastic changes.
Previous reports have emphasized that long-delayed recurrence is due to eradication of the high-grade immature component and the induction of a high degree of differentiation, thus enhancing the disease-free interval time [1]. The present case could be exceptional because the recurrent tumor showed much more mitoses and pronounced blastemal components than did the primary tumor, which cannot be explained by the chemotherapy-induced maturation phenomenon alone. The present case may indicate that chemotherapy does not always induce high-grade differentiation with long delayed recurrence, even though the chemoradiation therapy might have delayed the time of recurrence for an unknown reason. It is also possible that the chemoradiation therapy might have had little or no effect in our patient, because the therapy was given after the radical nephrectomy with no gross residual tumor.
The pathogenesis of late relapse in Wilms' tumor and in other neoplasms is not clearly understood, but several mechanisms have been suggested, such as escape from immune vigilance, scarce vascularization in some portion of the tumor, and cell dependency on growth factors [8]. The chemoradiation therapy itself cannot explain the complex mechanism of late-relapsing Wilms' tumors, and more studies are necessary to unveil the pathogenesis of late recurrence. Recent studies have indicated long-term survival after autologous stem cell rescue in Wilms' tumor, ranging from 40 to 73%. However, there are very few cases of long-delayed recurrence with stem cell transplantation. Brown et al. [6] reported a late recurrent case of Wilms' tumor that was successfully treated with high-dose chemotherapy and autologous stem cell rescue, resulting in a third complete response with a disease-free interval of 15 months post-transplant.
After surgery, the patient received 8 fractions of 1,440 Cgy radiation therapy on the whole abdomen and a chemotherapy POG 9444/CCG 4942 NWTS-5 regimen. She had autologous peripheral blood stem cell transplantation in July 2010 and has been free of recurrence or metastasis for 14 months.
In summary, we have reported a case of long-delayed recurrence in a 28-year-old female patient, 25 years after the primary diagnosis and treatment of a Wilms' tumor at the age of 3 years. This case to the best of our knowledge is the most delayed case of recurrence reported in the literature. This case illustrates that, in contrast with previously reported cases, a longer delayed recurrence may show more malignant features compared with the primary tumor.
Figures and Tables
FIG. 1
Computed tomographic finding showing a huge mass with enhancing solid portion involving almost the entire lower abdominal cavity.
FIG. 2
Grossly, the recurrent 30-cm mass had a yellowish pink to red, firm and lobulating cut surface with focal hemorrhage and necrosis.
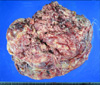
FIG. 3
(A) Microscopically, the recurrent tumor showed a classic triphasic growth pattern with blastemal, epithelial, and mesenchymal components (H&E, ×40). (B) Hypercellular blastemal element with adjacent tumor necrosis (H&E, ×40). (C) Short spindle mesenchymal cells in myxoid background (H&E, ×400). (D) Epithelial component with tubular growth pattern (H&E, ×400). (E) Undifferentiated blastemal cells with frequent mitoses (H&E, ×400).
References
1. Senetta R, Macrì L, Pacchioni D, Castellano I, Cassoni P, Bussolati G. Late recurrence of Wilms' tumour with exclusive skeletal muscle phenotype 23 years after primary diagnosis. Virchows Arch. 2007. 450:115–118.
2. Dome JS, Liu T, Krasin M, Lott L, Shearer P, Daw NC, et al. Improved survival for patients with recurrent Wilms tumor: the experience at St. Jude Children's Research Hospital. J Pediatr Hematol Oncol. 2002. 24:192–198.
3. Green DM. The treatment of stages I-IV favorable histology Wilms' tumor. J Clin Oncol. 2004. 22:1366–1372.
4. Li W, Kessler P, Yeger H, Alami J, Reeve AE, Heathcott R, et al. A gene expression signature for relapse of primary wilms tumors. Cancer Res. 2005. 65:2592–2601.
5. Sen S, Kadamba P, Al-AbdulAaly M, Mammen KE, Ahmed S. Results of Wilms' tumour management in two tertiary-care hospitals in Asia. Pediatr Surg Int. 1998. 13:42–44.
6. Brown E, Hebra A, Jenrette J, Hudspeth M. Successful treatment of late, recurrent wilms tumor with high-dose chemotherapy and autologous stem cell rescue in third complete response. J Pediatr Hematol Oncol. 2010. 32:e241–e243.
7. Gottesman JE, Pellettiere EV, Kilmer W. Recurrence of Wilms tumor-twenty-three years later. Urology. 1981. 17:268–269.
8. Gallego-Melcón S, Sánchez de Toledo J, Doste D, López D, Moraga F, Rodríguez C, et al. Late recurrent metastasis in Wilms' tumour. Med Pediatr Oncol. 1994. 23:158–161.
9. Pinarli FG, Oğuz A, Karadeniz C, Poyraz A, Konuş O, Citak C. Late relapsing Wilms tumor with rhabdomyomatous differentiation. Pediatr Blood Cancer. 2009. 52:675–677.
10. Gibson PJ, Vadeboncoeur CM, Johnston DL. Relapse of Wilms' tumor 13 years after original diagnosis. J Pediatr Hematol Oncol. 2005. 27:293–294.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download