Abstract
Purpose
Antimuscarinic therapy remains one of the most common forms of therapy for overactive bladder (OAB) in children. However, few clinical studies on the outcomes of antimuscarinics in children with OAB have been published. Therefore, we evaluated the efficacy and safety of propiverine, which is frequently prescribed for the treatment of pediatric OAB.
Materials and Methods
We retrospectively reviewed children with OAB treated with propiverine within the past 5 years. The response rates were compared between the non-urge incontinence (non-UI) and urge incontinence (UI groups). The cumulative response rate by treatment duration was also compared between the two groups.
Results
Among a total of 68 children, 50 children (73.5%) experienced UI. The overall response rate was 86.8%. Functional bladder capacity after treatment was 150 ml, which represented an increase compared with the value (140 ml) before treatment. The voiding frequency per day decreased from 14.0 to 8.5 times. The overall response rate (88.0%) in the non-UI group was not significantly different from that seen in the UI group (83.3%; p>0.05). In non-UI children, the cumulative response rates were 36.0%, 54.0%, 68.0%, 74.0%, 76.0%, and 78.0% at 4, 8, 12, 16, 20, and 24 weeks, respectively. The cumulative response rates in the UI children were 11.1%, 33.3%, 44.4%, 50.0%, 50.0%, and 55.6%, respectively during the same respective time periods. Adverse effects were identified in only two (2.9%) patients, and neither case was severe.
Overactive bladder (OAB) is relatively common in children. Although urotherapy, including lifestyle modifications, is recommended initially, antimuscarinic medication remains one of the most common forms of therapy for OAB in children. Although various antimuscarinic drugs have been introduced and are widely used with proven efficacy and stability in adult OAB patients, not all antimuscarinic drugs are suitable for children.
Oxybutynin chloride is the only antimuscarinic drug licensed for use in pediatric patients by the Food and Drug Administration in the United States. Most clinicians have prescribed oxybutynin for pediatric OAB patients. However, oxybutynin is now available in an extended-release tablet form, which is superior to the previous immediate-release tablet form but is still not recommended for infants and toddlers.
Propiverine, an antimuscarinic drug widely used in adult OAB patients, is popular in pediatric patients as well because it can be easily ground into a powdered form. In practice, propiverine could be easily administered to OAB children with better drug compliance. However, few clinical studies about the outcomes of propiverine in children with OAB have been reported. In the present study, therefore, we evaluated the efficacy and safety of propiverine.
Children with OAB who were treated with propiverine from October 2006 to February 2011 in Samsung Medical Center were retrospectively reviewed. This research protocol was approved by the Samsung Medical Center Institutional Review Board. A detailed history, including a lower urinary tract symptom (LUTS) questionnaire [1], physical examination, and 3-day frequency volume chart, was obtained from each patient. Urinalysis and uroflowmetry with residual urine check were also performed for each patient. Only children who received the drug for at least 2 weeks and who had evaluable pre- and post-treatment frequency volume charts were included in this study. Any children with neuropathic bladder, spinal dysraphism, anatomical abnormalities (e.g., posterior urethral valves or vesicoureteral reflux), or suspected urinary tract infections were excluded.
The efficacy of propiverine was measured on the basis of improvement of OAB symptoms (frequency, urgency, and urge incontinence), change of voiding frequency, and maximum voided volume. The symptom improvement data after propiverine treatment were classified into the following three categories: nonresponse (0 to 49% decrease of symptoms), partial response (50 to 99% decrease of symptoms), and full response (100% decrease of symptoms). Any adverse effects related to treatment were recorded.
Patients were divided into the non-urge incontinence (non-UI) and urge incontinence (UI) groups according to their symptom severity. The response rate was compared between the non-UI and UI groups. The cumulative response rates by treatment duration were also compared between the two groups.
To evaluate differences in treatment success between groups, we used the Pearson chi-square test. Data were analyzed by using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Probability values less than 0.05 were considered to be statistically significant.
During the study period, 68 children (37 male, 31 female) with a median age of 5.9 years (range, 3.1 to 10.0 years) satisfied the inclusion criteria. Eighteen patients (26.5%) had UI, 17 patients (25.0%) experienced nocturnal enuresis, and 4 patients (5.9%) had constipation at treatment entry. Median voiding frequency was 14 times per day (range, 8 to 72 times), and the median functional bladder capacity was 140 ml (range, 30 to 300 ml). The median treatment duration was 7.5 weeks (range, 2 to 66 weeks), and the propiverine dosage at the end of the treatment period was 10 mg for 25 children (36.8%) and 20 mg for 43 children (63.2%) (Table 1).
The median voiding frequency during the daytime decreased from 14.0 to 8.5, and median functional bladder capacity changed from 140 to 150 ml (Fig. 1). The overall response rate after propiverine treatment was 86.8% in all the children. The response rate was higher in the non-UI group (88.0%) than in the UI group (83.3%), but the difference was not statistically significant (p>0.05) (Fig. 2).
The symptom improvement rate increased with treatment duration. In non-UI children, the cumulative response rates were 36.0%, 54.0%, 68.0%, 74.0%, 76.0%, and 78.0% at 4, 8, 12, 16, 20, and 24 weeks, respectively. The cumulative response rates in UI children were 11.1%, 33.3%, 44.4%, 50.0%, 50.0%, and 55.6%, respectively, during the same period (Fig. 3). Adverse effects were identified in only two patients (2.9%), and neither case was severe (one case of dry mouth and one of constipation) (Table 2).
OAB is frequently seen in childhood. The incidence of OAB in school-aged children ranges from 17.8 to 26% [2-4]. In a recent study, the incidence of pediatric OAB in South Korea was 16.6% in children aged 5 to 13 years [5].
The etiology of OAB is not completely understood. The prevailing theory of pediatric OAB is a delay in the acquisition of cortical inhibition over uninhibited detrusor contractions. In general, it is believed that the maturation period for cortical control of voiding function is between 3 and 5 years of age, and the completion period for fine-tuning of the detrusor muscle is about 8 years [6]. The sites of maturational delay are thought to be the reticulospinal pathways of the spinal cord or the inhibitory center of the cerebral cortex [7]. Children with OAB might have difficulty achieving the mature voiding patterns that are characteristic of adulthood. Inflammatory processes in the bladder wall are also thought to be an etiology of pediatric OAB. Such inflammation can also irritate receptors in the submucosal and detrusor muscle layers and lead to LUTS [8].
Children with idiopathic OAB are initially treated with urotherapy; a nonsurgical, nonpharmacological treatment consisting of lower urinary tract rehabilitation using regular voiding with drinking and voiding charts, lifestyle changes, and some specific interventions such as physiotherapy, alarm therapy, and neurostimulation [9]. When urotherapy fails, antimuscarinic drugs can be used. For adult OAB patients, various antimuscarinic drugs such as oxybutynin, propiverine, tolterodine, trospium chloride, and solifenacin have been introduced and are widely used with proven efficacy and stability [10-14]. In contrast, oxybutynin chloride is the only FDA-approved antimuscarinic drug licensed for use in pediatric patients [15]. Currently, oxybutynin extended release (ER) rather than oxybutynin immediate release (IR) is widely administered in South Korea. Oxybutynin ER is superior to oxybutynin IR with regard to adverse effects; however, it is only available in tablet form, which is difficult for infants and toddlers to swallow. OAB children might show better drug compliance when treated with propiverine hydrochloride than oxybutynin because propiverine can be easily ground into a powdered form, whereas oxybutynin ER tablets cannot be broken [16]. In addition, a previous comparative study of propiverine and oxybutynin in children revealed lower adverse drug reaction rates for propiverine than oxybutynin [17]. Therefore, it is very important that clinicians understand the efficacy and safety of this drug, which inevitably could be used in clinical practice. Our study is meaningful because it is the first report of the efficacy and safety of propiverine in East Asian children. Recently, a randomized placebo-controlled clinical trial reported objective efficacy and good tolerability of propiverine [18]. Although that study was a well-designed randomized controlled study, it did not cover the differences in clinical outcomes according to the symptom severity of the OAB children. Our study is a comparative one of the efficacy of propiverine for symptom severity.
We found that propiverine therapy leads to a significant increase in functional bladder capacity and a decrease in voiding frequency. The drug also dramatically improved LUTS in OAB children; the overall response rate was 86.8% for the total treatment group. A previous study reported the overall efficacy of propiverine in OAB children to be 86.8%, and we found similar results in our study [17]. Propiverine is thought to act on the bladder via dual modes of action. Antimuscarinic agents cause parallel shifts to the right of the concentration-response curves to acetylcholine. In addition to this antagonistic muscarinic effect, which it shares with other antimuscarinic agents, propiverine causes a concentration-dependent inhibition of the KCl- and CaCl2-induced contractions [19].
Clinical practitioners have often encountered the problem of how long to prescribe anticholinergic drugs. According to our results, treatment should last for at least 8 weeks to achieve a response rate of 50%. For children with UI, the treatment period was longer than for those in the non-UI group. The response rate after 8 weeks of treatment was 54.0% for the non-UI group, 33.3% for the UI group, and 48.5% for the overall treatment group. To the best of our knowledge, this study was the first to evaluate the cumulative response rate of propiverine for OAB children without UI; it is also the first study that compared non-UI and UI groups. We believe that our results will provide advantageous information about propiverine for pediatric use in clinical practice.
Adverse effects were reported in only two patients, and neither case experienced severe symptoms. This finding supports previous clinical reports that children experience adverse effects (such as dry mouth or visual disturbances) less frequently than do adults when treated with propiverine [19]. Our data showed lower adverse events than did previous studies that reported adverse effect rates after anticholinergics treatment in OAB children [15-19]. Several hypotheses are possible to explain this difference. Propiverine might act specifically on the bladder in children; therefore, extravesical adverse effects are minimal. The other hypothesis is that children cannot easily express the symptoms of adverse reactions; thus, these might be underreported.
Our study had several limitations. First, it was an uncontrolled retrospective study, and we evaluated the efficacy of therapy by using frequency volume charts and subjectively described improvements alone. The potential for bias also existed in the patient selection because we included only children with relatively good drug compliance. The second limitation of our study was that most of our patients received medication therapy not by itself but combined with standard urotherapy. The improvement in OAB symptoms might be partially a result of the standard urotherapy. Despite these limitations, however, our study results contain important and practical information for antimuscarinic drug treatment, especially propiverine, in children with OAB.
Figures and Tables
FIG. 1
Changes in voiding frequency and maximum voided volume after propiverine treatment. (A) Change in voiding frequency. (B) Change in functional bladder capacity. UI, urge incontinence.

FIG. 2
Symptom improvement rate of propiverine treatment. Nonresponse, 0 to 49% decrease; Partial response, 50 to 99% decrease; Full response, 100% decrease in incontinence or less than 1 incontinence incident monthly. UI, urge incontinence.
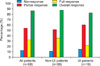
FIG. 3
Symptom improvement rate of treatment with respect to duration of treatment. UI, urge incontinence.

References
1. Kwak KW, Park KH. Clinical inconsistency of lower urinary tract symptoms between questionnaire and bladder diary in children with nocturnal enuresis. J Urol. 2008. 180:1085–1089.
2. Link CL, Lutfey KE, Steers WD, McKinlay JB. Is abuse causally related to urologic symptoms? Results from the Boston Area Community Health (BACH) Survey. Eur Urol. 2007. 52:397–406.
3. Hellström AL, Hanson E, Hansson S, Hjälmås K, Jodal U. Micturition habits and incontinence in 7-year-old Swedish school entrants. Eur J Pediatr. 1990. 149:434–437.
4. Kajiwara M, Inoue K, Kato M, Usui A, Kurihara M, Usui T. Nocturnal enuresis and overactive bladder in children: an epidemiological study. Int J Urol. 2006. 13:36–41.
5. Chung JM, Lee SD, Kang DI, Kwon DD, Kim KS, Kim SY, et al. Prevalence and associated factors of overactive bladder in Korean children 5-13 years old: a nationwide multicenter study. Urology. 2009. 73:63–67.
6. Franco I. Overactive bladder in children. Part 1: pathophysiology. J Urol. 2007. 178(3 Pt 1):761–768.
7. Saini R, Gonzalez RR, Te AE. Chronic pelvic pain syndrome and the overactive bladder: the inflammatory link. Curr Urol Rep. 2008. 9:314–319.
8. Steers WD. Pathophysiology of overactive bladder and urge urinary incontinence. Rev Urol. 2002. 4:Suppl 4. S7–S18.
9. Hellström AL. Pathophysiology ochildren with dysfunctional bladder. Scand J Urol Nephrol Suppl. 1992. 141:106–107.
10. Diokno AC, Appell RA, Sand PK, Dmochowski RR, Gburek BM, Klimberg IW, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003. 78:687–695.
11. Madersbacher H, Mürtz G. Efficacy, tolerability and safety profile of propiverine in the treatment of the overactive bladder (non-neurogenic and neurogenic). World J Urol. 2001. 19:324–335.
12. Kreder K, Mayne C, Jonas U. Long-term safety, tolerability and efficacy of extended-release tolterodine in the treatment of overactive bladder. Eur Urol. 2002. 41:588–595.
13. Zinner N, Gittelman M, Harris R, Susset J, Kanelos A, Auerbach S, et al. Trospium chloride improves overactive bladder symptoms: a multicenter phase III trial. J Urol. 2004. 171(6 Pt 1):2311–2315.
14. Chapple CR, Rechberger T, Al-Shukri S, Meffan P, Everaert K, Huang M, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004. 93:303–310.
15. Hoebeke P, De Pooter J, De Caestecker K, Raes A, Dehoorne J, Van Laecke E, et al. Solifenacin for therapy resistant overactive bladder. J Urol. 2009. 182:4 Suppl. 2040–2044.
16. Um JM, Kim KM. Efficacy and tolerability of extended-release oxybutynin in children with a neurogenic bladder. Korean J Urol. 2007. 48:1064–1068.
17. Alloussi S, Mürtz G, Braun R, Gerhardt U, Heinrich M, Hellmis E, et al. Efficacy, tolerability and safety of propiverine hydrochloride in comparison to oxybutynin in children with urge incontinence due to overactive bladder: results of a multicentre observational cohort study. BJU Int. 2010. 106:550–556.
18. Marschall-Kehrel D, Feustel C, Persson de Geeter C, Stehr M, Radmayr C, Sillén U, et al. Treatment with propiverine in children suffering from nonneurogenic overactive bladder and urinary incontinence: results of a randomized placebo-controlled phase 3 clinical trial. Eur Urol. 2009. 55:729–736.
19. Wada Y, Yoshida M, Kitani K, Kikukawa H, Ichinose A, Takahashi W, et al. Comparison of the effects of various anticholinergic drugs on human isolated urinary bladder. Arch Int Pharmacodyn Ther. 1995. 330:76–89.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download