Abstract
Purpose
The effects of partial nephrectomy (PN) on postoperative blood pressure (BP) are not known, and PN has the potential to worsen BP. We therefore sought to determine whether PN alters postoperative BP.
Materials and Methods
Patients who underwent PN for suspected malignancy at our institution from 2002 to 2008 were included. Data on BP and medication from before and after PN were retrieved from family physicians. BP and number of antihypertensive medications were compared after surgery with preoperative values by use of paired t tests and Chi-squared analyses, respectively.
Results
Of 74 patients undergoing PN and providing consent, 48 met the inclusion and exclusion criteria, with a median follow-up of 24 months. For the early postoperative period (1 month to 1 year after surgery), the mean BPs (132.3/77.0 mmHg) were unchanged compared with preoperative values (132.4/78.0 mmHg; p=0.59 systolic BP and p=0.30 diastolic BP). For the later postoperative period (beyond 1 year after surgery), the mean postoperative systolic BP was unchanged from the mean preoperative systolic BP (131.2 mmHg vs. 132.4 mmHg, respectively; p>0.30). However, the corresponding average diastolic BP was lower in the long term (78.0 mmHg versus 76.4 mmHg respectively; p=0.01). No significant difference in the mean number of BP medications prescribed preoperatively, at one year, and beyond one year was identified (p>0.37).
The management of small renal masses (up to 7 cm) has undergone a paradigm shift in the past 10 years from radical nephrectomy to renal sparing in the form of partial nephrectomy (PN), ablative interventions, and surveillance [1]. Several series have shown similar disease-specific survival and recurrence rates for PN compared with radical nephrectomy for renal masses smaller than 4 to 7 cm with the preservation of renal function [2-4]. Patients undergoing radical nephrectomy for renal masses tend to have a higher incidence of chronic renal insufficiency, cardiovascular events, and proteinuria than do patients undergoing PN [3,5-8].
Despite the advantage of preserving renal mass, the pathophysiological implications of PN are just beginning to be understood. PN has the potential to elicit a renin-angiotensin response in the treated kidney and thus hypertension. Hypothetically, renal artery injury from hilar clamping or the compressive effects of bolsters on the parenchymal defect after mass removal could mimic the Goldblatt one-clip two-kidney model of hypertension [9,10]. In short, reduced blood flow and glomerular capillary pressure in the affected kidney could cause renin release and ensuing hypertension. A handful of case reports have indicated that PN may precipitate postoperative hypertension in the short term [11-13]. One older small series of 14 patients showed no long-term deterioration of blood pressure (BP) after PN in patients with a solitary kidney, although that study did not include comparison of postoperative BP with preoperative BP [14]. The renal trauma literature suggests that renal injury can lead to postoperative hypertension in the form of renal vascular injuries or the often mentioned "Page kidney" after renal repair [15-17]. The donor transplant nephrectomy literature has conflicting studies with respect to the loss of renal mass on BP, with some showing a high incidence of hypertension after surgery [18,19] and one older study showing a minimal effect on long-term BP [20]. The importance of hypertension with respect to renal preservation is highlighted by a recent study identifying it as an independent risk factor for renal loss in radical and partial nephrectomies [7]. Therefore, the effect of PN for renal masses on postoperative BP is important for clinical follow-up and is not well defined.
The hypothesis suggesting that PN can induce a postoperative hypertensive response as a consequence of renin-angiotensin activation appears plausible. Therefore, our aim was to initiate an exploratory study to describe the effect of PN on short- and long-term postoperative BP.
A single-center, ethics-approved retrospective review of all PNs performed over 6 years (2002 to 2008) was conducted at the Queen Elizabeth II Health Sciences Center, Department of Urology in Halifax, Nova Scotia, Canada. The 6-year cut-off was selected because before this time period, PN was not a common procedure. Consent forms and explanatory cover letters were mailed to all 112 consecutive patients originally identified, and 74 provided informed consent. Of the 74 family physician offices contacted, 62 released information on BP and medications. Other patient information was also obtained from electronic and paper health records.
The inclusion criteria were patients ≥18 years of age, who underwent PN for presumed renal cell carcinoma, and who were followed by a family physician and had at least two BP readings taken during the 2 years before surgery and at least two BP measurements taken >1 month after surgery. The initial postoperative period was excluded to avoid effects of postoperative pain and anxiety on BP. Preoperative BP data were generated before patients were diagnosed with a renal cancer, thereby excluding anxiety-induced BP alterations. We excluded patients if consent was not given for information release or if insufficient BP information was available (less than two readings before or after surgery). Patients on dialysis within 2 years of surgery were excluded, thus eliminating the complexity of dialysis-related BP variability. Patients pregnant within 2 years before or after surgery were excluded. Patients with metastatic renal cancer within 2 years before or after surgery were excluded, because associated pain, anxiety, and paraneoplastic syndromes could complicate results. Patients with chronic renal failure (creatinine clearance <20 ml/h) and patients with greater than one renal surgery were also excluded. Of 62 patients available before exclusions, 10 had insufficient BP information, 2 developed early metastatic disease, 1 developed kidney failure, and 1 had bilateral kidney surgeries.
The main study variables assessed were mean systolic BP (SBP) and diastolic BP (DBP). The mean SBPs and DBPs were calculated separately before and after surgery. Unfortunately, owing to the retrospective nature of this study, we could not control for the temporal variability of BP readings before and after surgery. Therefore, BPs taken during the 2-year period before surgery were averaged, whereas the mean of BPs taken after surgery was stratified by the early postoperative period (1-12 months) and the late postoperative period (12 months to the end of follow-up). The secondary endpoint assessed was the mean number of BP medications used before (at the last family physician office visit before surgery) and after surgery. Postoperative BP medication tallies were also subdivided into the early and delayed postoperative period as above, with the number of medications at the last family physician visit closest to 1 year postoperatively and those at the last follow-up incorporated into our analysis. The number of patients taking angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) was compared independent of other medications.
Differences between mean SBPs and DBPs before and after surgery were analyzed with a paired t test. A p-value of <0.05 was considered statistically significant. Medication comparisons from before and after surgery were analyzed by using a chi-squared test. Statistical analysis was performed with GraphPad Prism ver. 4.01 (GraphPad Software Inc., La Jolla, CA, USA).
The characteristics of the patient cohort are summarized in Table 1. The overall mean preoperative SBP/DBP was 132.4/78.0 mmHg and when compared with the overall postoperative BP (132.2/76.7 mmHg) was not significantly different (p=0.87 for systolic and p=0.16 for diastolic). Table 2 shows the comparison of preoperative BP with BP in the early postoperative period (1 month-1 year) and the late postoperative period (>1 year). No significant difference was found in diastolic or systolic BP when preoperative measurements were compared with measurements in the early postoperative period (p≥0.3). Assessment of late postsurgery BP (>12 months after surgery) identified no significant difference in mean SBP compared with before surgery, with a median follow-up time of 24 months (p=0.34). However, the corresponding mean DBP was significantly lower in the delayed group (76.4 mmHg vs. 78.0 mmHg), with an absolute difference of 1.6 mmHg (p=0.01). The cohort was also stratified by the laparoscopic and open approach with no significant difference in postoperative BP noted in either group relative to preoperative BP (data not shown).
As depicted in Fig. 1, there was no significant difference between the mean number of BP medications prescribed preoperatively (1.2), at 12 months (1.4, p=0.38), and beyond 12 months (1.3, p=0.44). Furthermore, the number of patients prescribed ACEI and ARB anti-hypertensives did not differ significantly from preoperative to any postoperative time points (p=0.58), with a total of 3/32 patients undergoing a dosage increase in either of these medications.
This is the first series to determine the effects of PN on preoperative versus postoperative BP. With the use of BP measurements routinely performed by family physicians, we retrospectively compared preoperative and postoperative BPs in 48 patients with a follow-up of 2 years. In the total cohort, there was no significant change in mean SBP or DBP within 1 year after surgery. Beyond 1 year after surgery, a minor improvement in DBP (1.6 mmHg) occurred with no change in SBP. The improvement in DBP was probably not clinically significant. Therefore, BP in the short-term and long-term appears to be preserved after PN, although the possibility remains for an increase or decrease in BP in a cohort with a longer follow-up or more patients recruited. These findings reject the hypotheses suggesting that the technique of PN can induce a postoperative hypertensive response as a consequence of renin-angiotensin activation.
An alternative explanation is that renal masses themselves could cause compressive effects on surrounding renal parenchyma or generate paraneoplastic syndromes that result in hypertension. A recent study from Montenegro showed an improvement in hypertension in 24 patients undergoing radical nephrectomy for renal cell carcinoma [21]. The average preoperative BP in that study was particularly high (161/99 mmHg) compared with our study (132/78 mmHg), and no details on tumor size or methods of recording BP were provided. Given that all patients in the Montenegro study underwent a radical nephrectomy, it is likely that the patient population in that study had much larger tumors than did our cohort, which could have a stronger negative influence on BP through paraneoplastic or compressive effects on the renal parenchyma. A major strength of our study is that preoperative BP data were generated before the patients were diagnosed with a renal cancer to reduce any anxiety or stress-related impact on BP, which was not likely the case in the study from Montenegro.
Several transplant nephrectomy studies are contradictory to the Montenegro study and actually show an increase in BP after nephrectomy [18,19]. The donor nephrectomy patient population is different in that these patients are highly selected, are younger in age, have minimal baseline comorbidities, and lack the potential paraneoplastic or compressive effects of renal tumors. With respect to our study, the possibility remains that postsurgery BP elevation could be masked by preoperative hypertension caused by the renal mass (compressive effects or paraneoplastic syndrome). Indeed, one cause of hypertension (the renal mass) could have been replaced by another cause of hypertension (surgical factors stimulating angiotensin), thus balancing out the effect of PN on BP. Alternatively, hypertension may have been caused by the tumor, whereby removing the tumor improved the BP as noted by the improvement in DBP in the total cohort beyond 1 year and the trend toward improvement in SBP and DBP in those undergoing an open approach. However, the prevalence of preoperative hypertension in our study population (69%) was similar to the published Canadian prevalence of hypertension in this age group (66%) [22], which suggests that there was minimal if any effect of renal masses on the incidence of preoperative hypertension.
One drawback in our study design is a potential bias for selecting patients who are closely followed with BP measurements by family physicians. These patients may have tighter BP control with medications, which could lead to a false-negative effect on BP. We addressed this concern by tabulating the number of BP medications the patients were taking before and after surgery. There were no significant differences in the number of BP medications taken before and after surgery. We were only able to reliably track ACEI and ARB medication dosage changes and found no significant dosage changes, which suggests a low likelihood of significant dosage changes to other antihypertensives. Therefore, medication adjustments had a low chance of affecting our results. Unfortunately, owing to the retrospective nature of our study, we were unable to account for changes in medications other than antihypertensives that could also affect blood pressure.
Another limitation in our study was the lack of a control comparison group such as patients who underwent radical nephrectomy. However, because we compared patients in a pairwise fashion (preoperative to postoperative) and demonstrated no effect on BP, a control group would not have changed our conclusion with respect to our original hypothesis. In addition, the majority of patients who undergo PN in our institution have masses <4 cm, such that a radical nephrectomy cohort would not be an equivalent control group because these patients typically have much larger renal masses with more potential to impact BP. Another limitation of our study was the lack of control of BP measurements with respect to setting, time of day, and technique. However, by using a paired design such that the same family physician performed the measurement before and after surgery, we tried to minimize inter-observer variability.
This study, although exploratory, provides the first evidence that PN for small renal masses in a contemporary cohort is not likely a risk factor for worse BP outcome compared with before surgery. The lack of BP deterioration is particularly important in view of the recent evidence identifying hypertension as an independent risk factor for renal impairment after partial or radical nephrectomy [7]. Overall, these results should be generalizable with respect to the effect of PN on BP in the cohort of patients with renal masses less than 4 cm. A larger follow-up study is required to confirm these results.
Figures and Tables
FIG. 1
Mean number of medications taken per patient before surgery compared with at 1 year after surgery and beyond 1 year at the last follow-up (p>0.37).
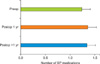
TABLE 1
Patient cohort characteristics

Values are presented as number (%) or median (range).
HTN, hypertension; DM, diabetes mellitus; lap, laparoscopic; AJCC, American Joint Committee on Cancer; SD, standard deviation; BP, blood pressure; IQR, interquartile range.
a: Comparison of open and lap warm ischemia time, p=0.037. b: AJCC Cancer Staging Manual, 7th ed., Reference [23], c: Comparison of preoperative to postoperative creatinine, p=0.43.
References
1. Gill IS, Aron M, Gervais DA, et al. Clinical practice. Small renal mass. N Engl J Med. 362:624–634.
2. Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001. 166:6–18.
3. Lau WK, Blute ML, Weaver AL, et al. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000. 75:1236–1242.
4. Dash A, Vickers AJ, Schachter LR, et al. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4-7 cm. BJU Int. 2006. 97:939–945.
5. Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. 2010. 183:1317–1323.
6. Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009. 181:55–61.
7. Malcolm JB, Bagrodia A, Derweesh IH, et al. Comparison of rates and risk factors for developing chronic renal insufficiency, proteinuria and metabolic acidosis after radical or partial nephrectomy. BJU Int. 2009. 104:476–481.
8. Zorn KC, Gong EM, Orvieto MA, et al. Comparison of laparoscopic radical and partial nephrectomy: effects on long-term serum creatinine. Urology. 2007. 69:1035–1040.
9. Johns C, Gavras I, Handy DE, et al. Models of experimental hypertension in mice. Hypertension. 1996. 28:1064–1069.
10. Ploth DW, Fitzgibbon W. Pathophysiology of altered renal function in renal vascular hypertension. Am J Kidney Dis. 1994. 24:652–659.
11. Uchida K, Takahashi A, Masumori N, et al. Partial nephrectomy for small localized renal cell carcinoma. Hinyokika Kiyo. 2004. 50:389–395.
12. Goel RK, Hickey LT, Rendon RA. Malignant hypertension due to renal artery stenosis after open partial nephrectomy in a solitary kidney. Urology. 2007. 69:385.e5–385.e7.
13. John J, Allen S, Perry M, et al. Page kidney phenomenon presenting as acute renal failure after partial nephrectomy: a case report and review of the literature. Urol Int. 2008. 80:440–443.
14. Novick AC, Gephardt G, Guz B, et al. Long-term follow-up after partial removal of a solitary kidney. N Engl J Med. 1991. 325:1058–1062.
15. Moudouni SM, Hadj Slimen M, Manunta A, et al. Management of major blunt renal lacerations: is a nonoperative approach indicated? Eur Urol. 2001. 40:409–414.
16. Nicol AJ, Theunissen D. Renal salvage in penetrating kidney injuries: a prospective analysis. J Trauma. 2002. 53:351–353.
17. Al-Qudah HS, Santucci RA. Complications of renal trauma. Urol Clin North Am. 2006. 33:41–53. vi
18. Azar SA, Nakhjavani MR, Tarzamni MK, et al. Is living kidney donation really safe? Transplant Proc. 2007. 39:822–823.
19. Fehrman-Ekholm I. Living donor kidney transplantation. Transplant Proc. 2006. 38:2637–2641.
20. Najarian JS, Chavers BM, McHugh LE, et al. 20 years or more of follow-up of living kidney donors. Lancet. 1992. 340:807–810.
21. Stojanovic M, Goldner B, Ivkovic D. Renal cell carcinoma and arterial hypertension. Clin Exp Nephrol. 2009. 13:295–299.
22. Onysko J, Maxwell C, Eliasziw M, et al. Large increases in hypertension diagnosis and treatment in Canada after a healthcare professional education program. Hypertension. 2006. 48:853–860.
23. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 2010. 7th ed. New York: Springer.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download