Abstract
Purpose
To investigate the correlation between functional bladder capacity, first morning urine osmolality, daytime voiding symptoms, and severity of nocturnal enuresis.
Materials and Methods
We assessed a total of 101 children with nocturnal enuresis (mean age, 7.7±2.3 years). Patients were divided into three groups according to the severity of enuresis: (1) one to six episodes per week (46 cases, 45.5%), (2) one episode every day (29 cases, 28.7%), and (3) multiple episodes every day (26 cases, 25.8%). Baseline parameters were obtained from frequency volume charts for 2 days, first morning urine osmolality, and a questionnaire for the presence of frequency, urgency, and daytime incontinence.
Results
The severity of enuresis increased with younger age (p=0.037) and reduced functional bladder capacity (p=0.007) and daytime symptoms of frequency and daytime incontinence (p=0.012, p=0.036). No statistical difference in urine osmolality or urgency was found among the three groups. Both reduced functional bladder capacity and low urine osmolality increased according to the severity of enuresis (p=0.012).
Conclusions
In children with nocturnal enuresis, severity was increased by younger age, reduced functional bladder capacity, and the presence of daytime voiding symptoms of frequency and daytime incontinence. The incidence of small functional bladder capacity was increased in children with everyday wetting, and the incidences of both small functional bladder capacity and low urine osmolality were increased in children with everyday multiple wetting.
Nocturnal enuresis (NE) is a common pediatric disease worldwide that affects 5.6% of children aged 5 to 13 years in Korea [1]. The simple explanation for NE is that nocturnal reservoir capacity is smaller than nocturnal urine output and difficult arousal due to deep sleep [2]. Although the evaluation and treatment of other causes of NE, including other voiding dysfunctions and constipation, upper airway obstruction and adenoid hypertrophy, attention deficit/hyperactivity disorder, and diabetes mellitus, are also important, treatment for NE targets the aforementioned three major factors. Under a recent guideline [3], primary treatment modalities of monosymptomatic NE (MNE) are alarm treatment and desmopressin, regardless of pathophysiology, rather than treatment based on causes. One important reason is that precise evaluation of the causes of enuresis is impractical and stressful, because convenient measuring of nocturnal bladder capacity and nocturnal urine output by use of a frequency volume chart cannot be applied to children with NE. Parents have to buy diapers and a precise electronic scale to measure the weight of the diapers to evaluate nocturnal bladder capacity and urine output. Because the guideline does not recommend evaluation of bladder capacity and urine output, many doctors only evaluate symptoms and conditions related to NE and voiding. Therefore, to evaluate the correlation between symptoms regarding NE, voiding, and causes of NE, we measured first voided urine osmolality for nocturnal polyuria and functional bladder capacity for nocturnal bladder capacity.
We assessed a total of 101 children with NE (mean age, 7.7±2.3 years) from September 2007 to July 2010. After a detailed individual history was taken, children taking any treatment or medicine for voiding dysfunction or NE in the previous 6 months were excluded. A physical examination including a neurologic examination and urinalysis was carried out. Baseline parameters were obtained from a frequency volume chart for 2 days, first morning urine osmolality as measured on a wetting day, and a questionnaire for the presence of frequency, urgency, daytime incontinence, and constipation [4].
Patients were divided into three groups according to the severity of enuresis: one to six episodes per week (group 1; 46 cases, 45.5%), one episode every day (group 2; 29 cases, 28.7%), and multiple episodes every day (group 3; 26 cases, 25.8%). Expected age-adjusted bladder capacity was calculated according to the formula: bladder capacity=12 (age [year] + 11) [5]. We regarded <65% of expected bladder capacity (EBC) as reduced functional bladder capacity (FBC) and >800 mOsm/kg as normal urine osmolality [6,7]. Bladder capacity and urine osmolality were classified as four types; type 1, normal FBC and normal urine osmolality; type 2, low urine osmolality but normal FBC; type 3, reduced FBC but normal urine osmolality; and type 4: reduced FBC and low urine osmolality.
Data were calculated with SPSS ver. 14 (SPSS, Chicago, IL, USA). Mann-Whitney U test, Chi-square test, and linear-by-linear test were used to evaluate the differences in FBC, urine osmolality, daytime urinary symptoms, and severity of enuresis between males and females. To evaluate differences in age, FBC, urine osmolality, and presence of daytime symptoms according to the severity of enuresis, the Kruskal-Wallis test and Chi-square test were used. Bivariate correlation analysis was applied to evaluate the correlation between the severity of enuresis and four types of causative two factors. A p<0.05 was considered statistically significant.
Among a total of 101 patients, 72 patients (73.1%) were male and 29 (28.7%) were female (Table 1). The mean bladder capacity for expected age was 71.9±30.8%, and it was reduced in 47 patients (46.5%). The bladder capacity of female patients (59.9±25.8%) was smaller than that of male patients (76.9±31.6%; p=0.011) (Table 2). The mean first morning urine osmolality was 868.1±215.2 mOsm/kg and it was low in 26 patients (25.7%) The urine osmolality of female patients (815.0±191.3 mOsm/kg) was lower than that of male patients (889.6±221.8 mOsm/kg; p=0.038). The incidence of urgency (56 cases, 55.4%) was almost twice that of frequency (31 cases, 30.7%) and urge incontinence (24 cases, 23.8%).
The severity of enuresis decreased with age (p=0.037) (Table 3). In a comparison of FBC and first morning urine osmolality according to the severity of enuresis, FBC was reduced according to the severity of enuresis (p=0.007), whereas first morning urine osmolality was not significantly different (p=0.774). Although the incidences of daytime frequency and urge incontinence were increased with the severity of enuresis (p=0.012, p=0.036), the incidence of urgency was not significantly different (p=0.178).
Small bladder capacity and low urine osmolality were positively correlated with the severity of enuresis (Pearson's correlation coefficient=0.249, p=0.012) (Table 4, Fig. 1). The most common type in group 1 was both normal FBC and urine osmolality (45.7%); group 2 was only small FBC and normal urine osmolality (48.3%). The incidence of both small FBC and low urine osmolality was higher in group 3 (30.8%) than in group 1 (4.3%) and 2 (6.9%) (Table 4).
The incidence of constipation was 12.9% (13/101 cases). The incidence of constipation in patients with daytime incontinence (25.0%, 6/24 cases) was higher than the incidence of no incontinence (9.1%, 7/77 cases) (p=0.042, Pearson's Chi square). Age, frequency, urgency, bladder capacity, urine osmolality and severity of NE were not correlated with constipation.
In the evaluation of NE by an evidence-based strategy, careful assessment of NE-related symptoms by questionnaire, physical examination, urine analysis, and frequency volume chart are only essential in the initial evaluation [8]. Furthermore, the recently published National Institute for Health and Clinical Excellence (NICE) guidelines do not recommend even urinalysis unless there is no suspicion of recent onset, daytime symptoms, urinary tract infection, or diabetes mellitus [3]. We were interested in determining the correlation between clinical symptoms evaluated by questionnaire and causes of NE that could be simply measured in real practice, because this may be helpful in the selection of a treatment modality.
To evaluate the presence and severity of enuresis, daytime urinary symptoms, constipation, and other related diseases, application of a specialized questionnaire is useful. The Korean questionnaire used in this study was developed by Kwak and Park [4], and has also been used as the official questionnaire by the Korean Children's Continence and Enuresis Society for the evaluation of patients with NE. The frequency volume chart is a useful noninvasive method for evaluating urine volume, daily urine output, and voiding patterns. In addition, maximum voided volume (MVV) is used as a descriptive factor for bladder accommodating ability. However, there is significant intra-individual variability in these measurements and different methods are applied in real practice, such as MVV after a holding maneuver and largest daytime voided volume (FBC) excluding first-morning volume as recorded on a frequency-volume chart [9,10]. FBC in a frequency volume chart can be as low as 50% of MVV [11].
In this study, 46.5% of a total of 101 patients had a reduced FBC for age, which was similar to other studies (30 to 50%) [2,11]. However, reduced FBC itself could not be regarded as a simple cause of NE, because NE occurs by mismatch of nocturnal urine output and bladder capacity [12].
The clinical significance of FBC or MVV in the management of NE is equivocal. In general, reduced FBC is a main factor not only in prediction of response to desmopressin but also refractory NE [13,14]. Eller et al. [14] reported that if FBC exceeds 70% of the age-adjusted norm, good response to DDAVP could be expected. With the other aspect of reduced bladder capacity, ratio of daytime to nocturnal bladder capacity is considered an important clinical factor. Kawauchi et al. [15] reported that a smaller bladder capacity during sleep than daytime functional bladder capacity, unlike in children without NE, may be an important cause of NE. However, Hansen et al. [9] found no significant difference between daytime and nighttime voiding volume. The result of the current study that reduced FBC significantly affected the severity of NE suggests that clinical features of reduced bladder capacity and overactivity in NE may be everyday wetting or multiple wetting, and perhaps early wetting after sleep.
Also, because bladder function can affect the presence of daytime urinary symptoms, evaluation of bladder function is important in patients with non-monosymptomatic NE (NMNE). In this study, daytime voiding symptoms were observed in more than half of the patients, and the prevalence of urgency was almost twice that of frequency and urge incontinence. In a Korean multi-center study, OAB was a significant risk factor in NE, and urgency was a better indicator of OAB than was frequency (>8 voidings per day) [16]. However, the results of the current study may reflect the difficulty in evaluating urgency in children. Because many normal children may feel urgency sometimes, which reflects a wide range of intra-individual variation, if a child has only urgency without frequency or incontinence, the possibility of voiding postponement should be considered. This makes the determination of true urgency indicating overactive bladder more difficult in children than in adults. Furthermore, in contrast to frequency and incontinence, which can be objectively evaluated by means of frequency-volume charts and wetting of underwear, urgency is difficult to evaluate objectively.
In the current study, low urine osmolality (>800 mOsm/kg H2O) was observed in 27.7% of patients, which is similar to the results of another study [17]. Although there is no standard normal-abnormal range of urine osmolality, because it rose more than 800 mOsm/kg H2O in the early morning under the influence of nighttime AVP secretion, 800 mOsm/kg was selected as a cutoff value in this study [10]. Although low osmolality at night is accompanied by increased urine volume in NE, there are several reports that spot urine osmolality is not predictive of response to desmopressin [17-19]. There is also a debatable opinion as to whether selection of treatment modality (DDAVP versus alarm) can be applied according to causative factors such as nocturnal polyuria or reduced FBC. Children with NE who have normal bladder function and capacity, fewer wet nights (<3 per week), only one enuretic event per night, age 8 years or more, and nocturnal polyuria are good candidates for treatment with desmopressin [2]. In contrast, children with reduced bladder capacity who void frequently in the daytime are candidates for alarm treatment [13,20]. However, Van Hoeck et al. [21] reported failure to identify high nocturnal urine output or a small-for-age nocturnal functional bladder capacity as a selection criterion for treatment.
Age was negatively correlated with the severity of NE in this study. Given that many children with NE will improve spontaneously, this result is not unexpected. However, age is one of the considerable factors in the selection of a treatment modality. The NICE guideline suggests an alarm for children under 7 years of age and desmopressin for children and younger people over 7 years of age. According to the results on severity of NE and grade of parameter in this study, the causes of NE cannot be clearly divided into two parameters, because some patients had a normal FBC and normal Uosm, and some patients had a small FBC and low Uosm. Although several studies have suggested clues as to which patients may be better candidates for alarm or desmopressin treatment [2,13,14], there is no suggestion of treatment for such patients. This result may also suggest that both parameters may be considered to be of relative value rather than of absolute value. Constipation is also an important factor that could negatively affect NE [22], and the presence of constipation was correlated with daytime incontinence in the current study.
There are several limitations to this study. First, the International Children's Continence Society has not proposed any grade of severity of enuresis [23] and recommends that the result of treatment should be specified from full response to nonresponse. Although the classification of severity of enuresis was also examined in a previous study [24], it is not generally used. The severity of enuresis in this study was determined subjectively under the assumption of bladder function between patients with only one wetting per day; the case may be different in patients with multiple wettings per day. However, the mean FBC/EBC in groups 2 and 3 was 70% and 58%, respectively, and the incidence of small FBC was similar (55.2% vs. 55.7%). This result may reflect the possibility of accompanying nocturnal polyuria with small FBC in children with everyday multiple wetting. The second important limitation in interpretation of this study is that daytime functional bladder capacity is not the same as the nocturnal situation, and urine osmolality is not directly correlated with nocturnal polyuria. If we want to apply cause-based treatment for NE, more precise evaluation methods for nocturnal detrusor overactivity and nocturnal polyuria will be needed. As another result of this study, the bladder capacity and urine osmolality of females were worse than the corresponding values for male patients; other studies reported no differences in the two parameters according to gender. This result may be due to selection bias, because of the relatively small number of female patients. Finally, this study included both MNE and NMNE. In general, the evaluation and treatment of MNE and NMNE differ. If a patient has symptoms of NMNE, more careful evaluation and different treatment is needed [3,6].
In children with nocturnal enuresis, severity is increased with younger age, reduced functional bladder capacity, and the presence of the daytime voiding symptoms of frequency and daytime incontinence. The incidence of small functional bladder capacity was increased in children with everyday wetting, and the incidences of both small functional bladder capacity and low urine osmolality were increased in children with everyday multiple wetting. However, because first morning urine osmolality is not a sensitive indicator of nocturnal polyuria, more precise evaluation methods for nocturnal detrusor overactivity and nocturnal polyuria will be needed to apply caused-based treatment for nocturnal enuresis.
Figures and Tables
FIG. 1
The four types (1 to 4) classified by the causative factors of functional bladder capacity and first morning urine osmolality according to the severity of nocturnal enuresis (Groups 1 to 3).
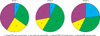
TABLE 2
Functional bladder capacity, first morning urine osmolality, daytime urinary symptoms, and severity of enuresis according to gender

References
1. Chung JM, Lee SD, Kang DI, Kwon DD, Kim KS, Kim SY, et al. An epidemiologic study of voiding and bowel habits in Korean children: a nationwide multicenter study. Urology. 2010. 76:215–219.
2. Hjalmas K. Gearhart JP, Rink RC, Mouriquand PD, editors. Nocturnal Enuresis. Pediatric urology. 2000. 1st ed. Philadelphia: Saunders;497–510.
3. Wootton J, Norfolk S. Nocturnal enuresis: assessing and treating children and young people. Community Pract. 2010. 83:37–39.
4. Kwak KW, Park KH. Clinical inconsistency of lower urinary tract symptoms between questionnaire and bladder diary in children with nocturnal enuresis. J Urol. 2008. 180:1085–1089.
5. Kim SO, Cho WY, Jung JM, Kim JM, Park SC, Kim YS, et al. Evaluation of functional bladder capacity in Korean children above 24 months old: a nationwide multicenter study. Korean J Urol Suppl. 2009. 50:112.
6. Nevéus T, von Gontard A, Hoebeke P, Hjälmås K, Bauer S, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children's Continence Society. J Urol. 2006. 176:314–324.
7. AbdelFatah D, Shaker H, Ismail M, Ezzat M. Nocturnal polyuria and nocturnal arginine vasopressin (AVP): a key factor in the pathophysiology of monosymptomatic nocturnal enuresis. Neurourol Urodyn. 2009. 28:506–509.
8. Hjalmas K, Arnold T, Bower W, Caione P, Chiozza LM, von Gontard A, et al. Nocturnal enuresis: an international evidence based management strategy. J Urol. 2004. 171:2545–2561.
9. Hansen MN, Rittig S, Siggaard C, Kamperis K, Hvistendahl G, Schaumburg HL, et al. Intra-individual variability in nighttime urine production and functional bladder capacity estimated by home recordings in patients with nocturnal enuresis. J Urol. 2001. 166:2452–2455.
10. Hamano S, Yamanishi T, Igarashi T, Murakami S, Ito H. Evaluation of functional bladder capacity in Japanese children. Int J Urol. 1999. 6:226–228.
11. Hagstroem S, Kamperis K, Rittig S, Djurhuus JC. Bladder reservoir function in children with monosymptomatic nocturnal enuresis and healthy controls. J Urol. 2006. 176:759–763.
12. Korzeniecka-Kozerska A, Zoch-Zwierz W, Wasilewska A. Functional bladder capacity and urine osmolality in children with primary monosymptomatic nocturnal enuresis. Scand J Urol Nephrol. 2005. 39:56–61.
13. Yeung CK, Sit FK, To LK, Chiu HN, Sihoe JD, Lee E, et al. Reduction in nocturnal functional bladder capacity is a common factor in the pathogenesis of refractory nocturnal enuresis. BJU Int. 2002. 90:302–307.
14. Eller DA, Austin PF, Tanguay S, Homsy YL. Daytime functional bladder capacity as a predictor of response to desmopressin in monosymptomatic nocturnal enuresis. Eur Urol. 1998. 33:suppl 3. 25–29.
15. Kawauchi A, Tanaka Y, Naito Y, Yamao Y, Ukimura O, Yoneda K, et al. Bladder capacity at the time of enuresis. Urology. 2003. 61:1016–1018.
16. Chung JM, Lee SD, Kang DI, Kwon DD, Kim KS, Kim SY, et al. The prevalence and risk factors of overactive bladder in Korean children: a comparative analysis according to definition. Korean J Urol. 2008. 49:1131–1139.
17. Kawauchi A, Watanabe H, Miyoshi K. Early morning urine osmolality in nonenuretic and enuretic children. Pediatr Nephrol. 1996. 10:696–698.
18. Folwell AJ, Macdiarmid SA, Crowder HJ, Lord AD, Arnold EP. Desmopressin for nocturnal enuresis: urinary osmolality and response. Br J Urol. 1997. 80:480–484.
19. Van Hoeck KJ, Bael A, Lax H, Hirche H, Bernaerts K, Vandermaelen V, et al. Improving the cure rate of alarm treatment for monosymptomatic nocturnal enuresis by increasing bladder capacity--a randomized controlled trial in children. J Urol. 2008. 179:1122–1126.
20. Djurhuus JC, Rittig S. Current trends, diagnosis, and treatment of enuresis. Eur Urol. 1998. 33:Suppl 3. 30–33.
21. Van Hoeck K, Bael A, Lax H, Hirche H, Van Dessel E, Van Renthergem D, et al. Urine output rate and maximum volume voided in school-age children with and without nocturnal enuresis. J Pediatr. 2007. 151:575–580.
22. Halachmi S, Farhat WA. The impact of constipation on the urinary tract system. Int J Adolesc Med Health. 2008. 20:17–22.
23. Nevéus T, von Gontard A, Hoebeke P, Hjälmås K, Bauer S, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children's Continence Society. J Urol. 2006. 176:314–324.
24. Stone J, Malone PS, Atwill D, McGrigor V, Hill CM. Symptoms of sleep-disordered breathing in children with nocturnal enuresis. J Pediatr Urol. 2008. 4:197–202.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download