Abstract
Spontaneously ruptured renal cell carcinoma (RCC) in end-stage kidney disease is very rare. Preoperative diagnosis is difficult because of the relatively small tumor size, associated hematoma, and surrounding acquired cysts. Two middle-aged men who were maintained on hemodialysis (HD) for over 10 years suddenly developed flank pain during HD. Computed tomography scans revealed an enhancing ruptured renal mass in one patient, and no obvious tumor lesion except for a hematoma in the other, both of which were later confirmed as RCCs by pathologic specimens.
Spontaneously ruptured renal cell carcinoma (RCC) is a well-known entity; however, such occurrences in patients with end-stage kidney disease are very rare [1]. To our knowledge, only 5 cases have been reported in the literature, all of which were associated with acquired cystic disease of the kidney (ACDK) [2]. Preoperative diagnosis is difficult because of the relatively small tumor size, associated hematoma, and surrounding acquired cysts. Herein, we report two cases of spontaneously ruptured RCC in patients with end-stage kidney disease, with a review of the current medical literature. In the first case, a ruptured enhancing tumor could be identified on the preoperative computed tomography (CT) scan that was not associated with ACDK. In the second case, only a hematoma with ACDK changes was detected by CT scan, but the patient was later confirmed to have RCC on microscopic examination of the pathologic specimen.
A 47-year-old male with end-stage renal disease (ESRD) who was maintained on regular hemodialysis (HD) for over 15 years presented with acute left flank pain during HD. His vital signs were stable, and a physical exam showed left costovertebral angle tenderness. Laboratory evaluation revealed a hemoglobin value of 7.3 g/dl, which was decreased by 2 points compared with his baseline value. No anti-coagulatory agents were included in his outpatient medications. An enhanced abdominal CT scan revealed a 3.2x2.5 cm sized ill-defined lobulated mass in the lower pole of the left kidney, which had acute extravasation of contrast material, probably due to tumor rupture (Fig. 1). An associated hematoma was also seen in the left retroperitoneum, and the contralateral right kidney was atrophied without cystic changes. Given the concern for a ruptured renal tumor, an open radical nephrectomy was performed. Gross section revealed a yellowish mass in the lower pole. The mass surface was previously ruptured and measured 3.2x2.5 cm. It had spread near the renal pelvis but did not involve the pelvocaliceal cavity and was limited to the kidney (Fig. 2A), which was compatible with clear cell type RCC, Fuhrman grade 1 on microscopic exam (pT1a). The patient was discharged 10 days after surgery without any complications.
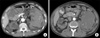
A 49-year-old male with ESRD visited our emergency center complaining of acute onset of left flank pain during HD. He had been maintained on HD for 10 years. His vital signs were stable upon arrival and a physical exam showed left costovertebral tenderness. The initial hemoglobin and hematocrit were 7.5 g/dl and 22.8%, respectively. No anti-coagulatory agents were included in his outpatient medications. An enhanced abdominal CT scan showed a 10x7.2x13 cm hyperdense hematoma with severe perirenal fat infiltration and fluid collection in the left retroperitoneum. However, no solid tumor could be identified in the area of contrast material extravasation. Multiple variably sized cysts were found in both kidneys, consistent with ACDK (Fig. 3A). Left renal angiography and renal arterial embolization were performed, but neither procedure revealed any aberrant or bleeding vessels. During the 2 weeks after the procedure, the patient's hemoglobin and hematocrit continued to decrease (6.2 g/dl) with increasing flank pain. A follow-up CT scan displayed a hematoma with a slightly increased size compared with the initial study (Fig. 3B), and as a result, an open nephrectomy was performed under the impression of persistent renal bleeding. The gross specimen revealed massive intrarenal hematomas without a solid mass (Fig. 2B). However, microscopic exam showed clear cell type RCC, with Fuhrman's nuclear grade 1 at the bleeding site in the mid portion (pT1a). The patient was discharged 8 days after the surgery without complications.
Spontaneous rupture of the renal parenchyma is rare and usually represents a complication of a serious underlying illness. Of the major causes reported, the most common etiology for spontaneous renal hemorrhage is a benign or malignant neoplasm (61%), with angiomyolipoma being predominant (29%) followed closely by RCC (26%). Vascular disease is the next most common offender (17%), with polyarteritis nodosa occurring most frequently [3]. The mechanism of spontaneous rupture of a renal tumor is not understood, but some authors have suggested that extension of the renal vein caused by elevations in venous pressure secondary to tumor emboli may be the cause. Others advocate that the rupture is related to the direct tumor invasion of the renal capsule or vessels [4].
A CT scan is the most valuable tool for examination of suspected spontaneous perirenal hemorrhage. Results of a meta-analysis showed that CT is 100% sensitive for the presence of retroperitoneal hemorrhage and has a higher sensitivity and specificity than ultrasound for identification of an underlying renal mass [3]. In addition, the identification of fatty elements in a renal mass on CT scans permits the specific diagnosis of angiomyolipoma, obviating the need for additional diagnostic measures. When no tumor is suspected on the basis of a CT scan, some have proposed conservative treatment with angiographic embolization [5]. This procedure has the ability to embolize bleeding vessels selectively, takes advantage of functional renal parenchyma preservation, and, potentially, circumvents the need for surgery and anesthesia [5].
On the contrary, small renal tumors may escape detection on radiographic studies, and there is no correlation reported between the tumor size and the frequency of spontaneous ruptures [6]. Poor perfusion of the renal vascular bed secondary to arterial spasm, infarction, or compression of intrarenal vessels by the hematoma may prevent arteriography from defining tumor vascularity. Even renal tumors less than 1 cm in diameter can spontaneously rupture [6]. Therefore, we should not exclude the possibility of a tumor rupture on the basis of negative imaging studies. Some clinicians have proposed nephrectomy in cases of unexplained spontaneous kidney ruptures due to the relatively high rate of RCC [7].
Spontaneously ruptured RCC in end-stage kidney disease is very rare. To our knowledge, there are only 5 cases reported in the literature, all of which were in Japanese men [2]. In those reports, all ruptured tumors occurred in patients with ACKD, and the tumor sizes were relatively small (microscopy only; 5.5 cm in diameter). Preoperative diagnosis of the cause of spontaneous renal rupture could not be made through routine imaging study in all cases because of the associated hematoma and surrounding acquired cysts [2].
The incidence of renal cancer in ESRD patients is higher than in the general population. Denton et al reported that the incidence of RCC in these patients was up 4.2% [8]. In addition, it is often difficult to diagnose renal tumor rupture in ACKD, because the tumor is covered by a variable cystic structure in most cases. Moreover, patients with ESRD have nonfunctioning kidneys. We believe that these 3 points: 1) a high incidence of RCC, 2) difficulty in diagnosis, and 3) nonfunctioning kidneys - strongly support the need for nephrectomy in cases of unexplained spontaneous kidney rupture in ESRD patients with ACKD. Even if considering conservative treatment, serial CT scans should be included either until complete resorption of the hematoma is visualized or a diagnosis is made [9].
Additionally, our patients both experienced their first symptoms during HD. We believe that the heparinization performed during HD may contribute to spontaneous ruptures. Renal cell neoplasms accompanied by ACDK are frequently bilateral (9%) [10]. Some authors have proposed the necessity of prophylactic bilateral nephrectomy, but controversy exists given the invasiveness of surgery and its associated complications.
Figures and Tables
FIG. 1
Case 1. Enhanced Computed tomography scan revealed a 3.2×2.5 cm sized ill-defined enhanced mass in the lower pole of the left kidney (arrow), which had acute extravasation of contrast material.
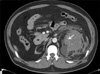
FIG. 2
Gross section. (A) Case 1. A partially ruptured yellowish mass in the lower pole of the kidney (arrow). (B) Case 2. Massive intrarenal hematomas without visible solid mass.
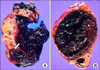
FIG. 3
Case 2. (A) Enhanced Computed tomography (CT) scan showed a 10×7.2×13 cm hyperdense hematoma in the left retroperitoneum; however, no solid tumor could be identified in the area of contrast material extravasation. (B) 2 weeks after follow-up enhanced CT scan displayed increased size (8.7×12×13 cm) of hematoma with improved prominent tortuous vascular structure.
References
1. Koh DH, Kim SJ, Ahn HS. Clinical analysis of spontaneous renal rupture with perirenal hemorrhage. Korean J Urol. 2004. 45:64–68.
2. Goto T, Sengiku A, Sawada A, Shibasaki N, Ishitoya S, Okumura K. Bilateral renal cell carcinoma of dialysis patient manifesting as spontaneous renal rupture. Hinyokika Kiyo. 2009. 55:707–710.
3. Zhang JQ, Fielding JR, Zou KH. Etiology of spontaneous perirenal hemorrhage: a meta-analysis. J Urol. 2002. 167:1593–1596.
4. Polky HJ, Vynalek WJ. Spontaneous nontraumatic perirenal and renal hematomas. Arch Surg. 1933. 26:196–218.
5. Koo V, Duggan B, Lennon G. Spontaneous rupture of kidney with peri-renal haematoma: a conservative approach. Ulster Med J. 2004. 73:53–56.
6. Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbetter WF. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971. 28:1165–1177.
7. Novicki DE, Turlington JT, Ball TP Jr. The evaluation and management of spontaneous perirenal hemorrhage. J Urol. 1980. 123:764–765.
8. Denton MD, Magee CC, Ovuworie C, Mauiyyedi S, Pascual M, Colvin RB, et al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int. 2002. 61:2201–2209.
9. Bosniak MA. Spontaneous subcapsular and perirenal hematomas. Radiology. 1989. 172:601–602.
10. Truong LD, Krishnan B, Cao JT, Barrios R, Suki WN. Renal neoplasm in acquired cystic kidney disease. Am J Kidney Dis. 1995. 26:1–12.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download