Abstract
Purpose
Many studies have been carried out to increase the success rate of shock wave lithotripsy (SWL) and to reduce renal injury. We investigated the success rate after one session as well as urine N-acetyl-β-d-glucosaminidase (NAG) levels for the evaluation of renal injury according to shock wave frequency and pretreatment with low-energy shock waves during SWL.
Materials and Methods
The study targeted 48 patients with renal stones who had undergone SWL. Patients were sequentially allocated into four groups according to shock wave frequency (60 or 120 shocks/min) and whether pretreatment had occurred. We documented total SWL operating number, success rate after first SWL, urine NAG, compliance, and the total cost for each patient.
Results
There were 32 males and 16 females with an average age of 51.6 years. The average stone size was 7.06 mm, and there was no significant difference in stone size between the groups. The data showed that patients treated with a frequency of 60 shocks/min had a lower mean number of SWL sessions, 1.36 sessions for 60 shocks/min and 2.0 sessions for 120 shocks/min, respectively, which was statistically significant (p<0.05). When comparing NAG/creatinin ratios before and after SWL between those with and without pretreatment, there was no significant difference according to pretreatment (p=0.406).
Conclusions
SWL treatment at a frequency of 60 shocks/min yielded better outcomes, such as a lower number of SWL sessions, and had an increased success rate compared with SWL at 120 shocks/min. On the other hand, pretreatment did not impact renal injury. Therefore, SWL treatment at a frequency of 60 shocks/min could improve treatment efficacy more than that for SWL at 120 shocks/min.
Since its introduction in the early 1980s, extracorporeal shock wave lithotripsy (ESWL) has been the most popular method for urinary stone treatment because it is relatively noninvasive, has few complications, and has a high success rate [1]. In particular, it has been the method of choice for treatment of renal stones ≤20 mm [2].
Although shock wave lithotripsy (SWL) is noninvasive, shock waves can cause renal injury in an area of intraparenchymal hemorrhage in the line of the blast path. Complications such as subcapsular and retroperitoneal hemorrhage have been well-recognized, and long-term issues such as hypertension, particularly in older patients, continue to be of concern. These complications have arisen from damage to the renal parenchyma, which is directly related to the number of shock waves, maximum kV administered [3], and the rate of voltage escalation during SWL treatment [4]. If slower shock wave rates lead to clinically improved stone fragmentation, patients could be at decreased risk for these side effects and be spared additional SWL or more invasive surgical treatments. To generate better results and fewer complications, many methods have been introduced, including elevation of shock wave power (voltage stepping), the use of lower frequency shock waves, and pretreatment with low-energy shock waves while performing SWL [4-6].
Greenstein first suggested improvements in stone fragmentation with a reduction in the frequency of shocks per minute, and following this report, many studies both in vitro and in vivo have reported the advantage of low-frequency shock waves [5,7,8]. Furthermore, Willis et al reported that renal injury could be reduced by pretreatment with low-energy shock waves during SWL [9].
N-Acetyl-β-d-glucosaminidase (NAG) is a lysosomal enzyme that is abundantly present in the cells of the proximal tubule and that has been considered a very sensitive marker of renal tubular impairment [10-12]. Also, the urine NAG/creatinine (Cr) ratio has been shown to increase after SWL and to remain high for 4 days after, requiring 4 weeks to normalize [13].
Many studies on SWL have used the Dornier Compact Delta lithotripter, LithoTron, and the Lithotripter S. We hypothesized that lower frequency shock waves and pretreatment with low-energy shock waves during SWL would generate better results and fewer complications related to SWL in renal stone patients being treated with a Sonolith Praktis (EDAP Technomed, France) in our hospital. In addition, we investigated the success rate after one session as well as urine NAG levels for the evaluation of renal injury according to shock wave frequency and pretreatment with low-energy shock waves during SWL, respectively.
In this case-controlled study, we prospectively audited consecutive adult patients who had undergone SWL for renal stones ≤15 mm in size from December 2010 to May 2011 in our hospital. We selected 48 patients who were diagnosed with renal stones and received SWL as the first treatment. These patients were sequentially allocated and assessed according to treatment success as measured by the stone-free rate and the degree of renal injury. We excluded patients with severe hydronephrosis, ureteral stricture, neurogenic bladder, multiple renal stones, or calyceal diverticular stones and those lacking follow-up. The diagnosis of renal stones was done with KUB, intravenous pyelography (IVP), or computed tomography (CT). Renal stone size was measured by the largest diameter following patient examination. We performed KUB for radio-opaque stones as a follow-up for renal stones at 2 week intervals.
Patients were sequentially treated by four methods. The first involved having been pretreated with low-energy shock waves during ESWL and having received 2,000 to 3,000 shocks at a rate of 60 shocks/min. The second method involved not having been subjected to pretreatment with low-energy shock waves and having received 2,000 to 3,000 shocks at a rate of 60 shocks/min. The third method involved having been subjected to pretreatment with low-energy shock waves and having received 2,000 to 3,000 shocks at a rate of 120 shocks/min. The fourth method involved not having been subjected to pretreatment with low-energy shock waves and having received 2,000 to 3,000 shocks at a rate of 120 shocks/min.
Patients were allocated into four groups for this study. Group I had received 2,000 to 3,000 shocks at a rate of 60 shocks/min, and group II had received 2,000 to 3,000 shocks at a rate of 120 shocks/min. All SWL was performed by individuals whom had performed at least 2,000 shocks. Furthermore, SWL was terminated when the treating urologist agreed that the stone appeared to be fragmented or when 3,000 shocks had been administered. Group III had been subjected to pretreatment with low-energy shock waves during SWL, and group IV had not been subjected to pretreatment with low-energy shock waves during SWL.
All SWL was done by using the Sonolith Praktis (EDAP Technomed, France) with the patient in a supine position. A session of SWL was set to 2,000 to 3,000 shocks. For pretreatment with low-energy shock waves, patients received 500 shocks of a 12 kV shock wave followed by 3 minutes of waiting time. The power of the shock wave was increased by 12 kV to a maximum of 24 kV [9]. In the non-pretreatment with low-energy shock waves group, the potency was increased from 12 kV to 24 kV. All procedures were performed under intravenous injection (IV) of ketorolac tromethamine 30 mg/ml to control pain. In cases of failure regarding pain control, we injected pethidine HCL 50 mg IV. After treatment, we advised all patients regarding oral hydration and exercise and checked for complications including pain, infection, hematuria, and the presence of D-J stenting.
Success was defined as asymptomatic status, stone-free status, or detection of asymptomatic residual stone fragments ≤3 mm in size [14]. To confirm success and excretion of renal stone fragments (stone-free status) after SWL, patients were assessed with KUB after 2 weeks. For nonvisible stones or minimal fragments on KUB, we confirmed success by noncontrast CT (NCCT) [15].
To investigate the influence of shock waves on kidney injury, each patient was evaluated with a complete blood cell count (CBC), blood urea nitrogen (BUN), creatinine, and urine NAG before lithotripsy and at 3 days after SWL. Because of differences in renal excretion function, urine NAG was calibrated by using urine Cr levels.
We compared groups I and II to evaluate the success rate of shock wave frequency variation. A comparison of groups III and IV was done to evaluate renal injury following pretreatment with low-energy shock waves.
We documented gender, age, stone size, stone location, number of sessions up to stone-free status, pain scale, Cr, NAG, NAG/Cr ratio, and total cost in each patient. Data were analyzed via a Student's t-test, ANOVA, and chi-square test using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at a level of 5%.
Of patients who had SWL treatment, we targeted 48. Their average age was 51.6 years, and patients were allocated into four groups as follows: 60 shocks/min, 120 shocks/min, and the presence or absence of low-voltage pretreatment shock waves. There were 32 males and 16 females, and an average stone size of 7.06 mm. The mean number of SWL sessions to success was 1.66.
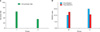
We performed a comparative analysis between the 60 shocks/min (group I) and 120 shocks/min (group II) groups to determine success rate after lithotripsy. Stone size, stone side, and stone location showed no significant differences between the two groups. The mean number of SWL sessions was 1.36 at a frequency of 60 shocks/min and 2.0 at a frequency of 120 shocks/min, a statistically significant difference (p=0.008). With respect to the success rate after the first treatment, 64.0% of patients were successfully treated in the 60 shocks/min group and 34.8% of patients were successfully treated in the 120 shocks/min group (p<0.05) (Table 1 and Fig. 1).
Results according to NAG/Cr levels after SWL treatment were assessed to investigate renal injury. Stone size, stone side, and stone location showed no significant differences between the two groups. When comparing NAG/Cr levels before SWL treatment with or without pretreatment with low-energy shock waves, groups III and IV exhibited no statistical differences. The data showed that NAG/Cr levels were, respectively, 12.61 (pretreatment with low-energy shock waves) and 10.53 (non-pretreatment with low-energy shock waves) after SWL treatment, a difference that failed to achieve statistical significance (p=0.406) (Table 2 and Fig. 1).
With regard to post-SWL complications, gross hematuria was frequent in 31 patients (65%), intolerable pain with ketorolac tromethamine 30 mg/ml in 18, and urinary tract infection in another. Furthermore, D-J stenting as an auxiliary procedure was required in 3 (6%). There were no significant differences between the groups.
Since its introduction in the 1980s, SWL has been used as the most popular method for urinary stone treatment because of its relatively high success rate and stability [1]. According to recent improvements in imaging devices, renal stone incidence has increased and the role of SWL has grown in importance. Moreover, a need has arisen for safer and more effective results with respect to SWL.
Recently, many studies have been conducted to improve the success rate through the use of effective analgesics, alpha- 1 adrenaline receptor antagonists, and a reduction in the frequency of shock waves when performing SWL [4-6]. A suitable number and frequency of shock waves for urinary stones has yet to be defined; however, according to a recent study, high-frequency shock waves can reduce stone fragmentation during lithotripsy. Yilmaz et al in a clinical setting, allocated patients into three groups: 60, 90, and 120 shocks/minute and found that success rates were similar between the groups receiving 60 and 90 shocks/minute and that both were higher than in the group receiving 120 shocks/minute [8]. Semins et al reported that when comparing 60 and 120 shocks/min, there was significant improvement in success rates [16]. Furthermore, Koo et al showed that 70 impulses/min compared with 100 significantly improved the success rate and reduced total cost [14].
According to our results, 60 shocks/min (group I) compared with 120 (group II) showed a significant improvement in the success rate. Furthermore, there were significant differences between 60 and 120 shocks/min with respect to the total number of SWL sessions and total cost. These results relate to the ability of low-frequency shock waves to improve the stone success rate and thus significantly affect the effective excretion of the renal stone. Additionally, the frequency of complications did not show any significant difference between the groups.
Many studies have supported the assertion that slower shock wave rates may improve stone comminution [17,18]. The mechanism for the increased efficiency of SWL at 60 shocks/min may be related to decreased acoustic impedance mismatch, improved cavitation bubble production on the stone surface, or improved bubble dynamics due to water and gas content surrounding the stone [19]. The most likely explanation has been related to the impact of the shock wave rate on cavitation bubbles. Although cavitation bubbles in contact with the stone surface contribute to stone fragmentation, more remote cavitation bubbles may act as a barrier to efficient shock wave energy transmission. Therefore, slowing the shock wave rate may allow this barrier of bubbles to dissipate, allowing increased gas content in the fluid medium adjacent to the stone, and support better cluster bubble dynamics on the stone surface to promote superior fragmentation.
Renal complications can be subdivided into early effects on kidney anatomy that lead to hematuria and hematoma formation and late complications that affect kidney function and cause systemic hypertension. Histopathological examination of human and animal kidneys has shown endothelial cell damage to midsized arteries, veins, and glomerular capillaries immediately after SWL [20,21]. Thin-walled arcuate veins in the corticomedullary junction have been shown to be especially vulnerable to shock wave exposure and are related to hematuria and hematoma [20].
SWL-induced acute renal damage may also result in severe injury to the nephron, microvasculature, and the surrounding interstitium [22]; renal tubules and vessels have been demonstrated to be more vulnerable than renal blood flow to discharge energy [23]. These injuries may be related to the long-term effects of SWL on renal function.
In an in vivo study, Wills et al reported that 500 pretreatments with low-energy shock waves reduced renal injury. It has been suggested that the low-energy shock waves cause vasoconstriction of vessels into the kidney and reduction of blood flow, resulting in an impact on the reduction of hematoma and renal injury during SWL [9].
In our study, we investigated NAG/Cr levels, Cr, and NAG released into urine during the early stages of renal cell injury in order to assess renal injury. As the final outcome, we did not find any significant difference in NAG/Cr levels between patients who had been pretreated with low-energy shock waves (group III) and those who had not been pretreated (group IV). This indicated that pretreatment before SWL does not affect renal injury. Our results showed that group IV had a lower NAG/Cr level than did group III.
Our study was not related to renal injury and pretreatment with low-energy shock waves; however, our study included a small number of patients, and NAG activity was high during the first 24 hours after lithotripsy in another study [24]. Additionally, if we had measured the number of patients and evaluated other renal enzymes, we may have gotten different results.
We first compared the success rate and renal injury simultaneously in relation to lower frequency shock waves and pretreatment with low-energy shock waves. We found that low-frequency shock waves improved the stone success rate and thus significantly affected the effective excretion of renal stones. In this study, no group exhibited an association between renal injury according to pretreatment with low-energy shock waves during ESWL; however, the success rates of low-frequency shock waves increased and the total ESWL sessions required decreased. Therefore, low-frequency shock waves may be helpful in preventing long-term complications according to reduced total ESWL numbers.
A limitation of this study was that we examined only 48 patients from a single center. To confirm our findings, further study should be carried out on a large scale. Such study should assess how variations in shock wave strength and pretreatment with low-energy shock waves affect the success rate and renal injury.
Shock wave lithotripsy treatment at 60 shocks/min yielded better outcomes of lower SWL sessions and increased success rate compared with treatment at a frequency of 120 shocks/min. In contrast, pretreatment with low-energy shock waves did not significantly impact renal injury. Therefore, SWL treatment at a frequency of 60 shocks/min could improve the treatment efficacy of the commonly used 120 shocks/min procedure. Further assessment of the relationship between pretreatment with low-energy shock waves and reduction in renal injury is suggested for further research.
Figures and Tables
FIG. 1
(A) Treatment outcomes according to frequency (p=0.044). (B) N-Acetyl-β-d-glucosaminidase (NAG)/creatinine (Cr) levels according to pretreatment (p=0.406). I: 60 shocks/min (group I), II: 120 shocks/min (group II), III: pretreatment (group III), IV: non-pretreatment (group IV).
References
1. Skolarikos A, Alivizatos G, de la Rosette J. Extracorporeal shock wave lithotripsy 25 years later: complications and their prevention. Eur Urol. 2006. 50:981–990.
2. Albala DM, Assimos DG, Clayman RV, Denstedt JD, Grasso M, Gutierrez-Aceves J, et al. Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis-initial results. J Urol. 2001. 166:2072–2080.
3. Delius M, Enders G, Xuan ZR, Liebich HG, Brendel W. Biological effects of shock waves: kidney damage by shock waves in dogs--dose dependence. Ultrasound Med Biol. 1988. 14:117–122.
4. Zhou Y, Cocks FH, Preminger GM, Zhong P. The effect of treatment strategy on stone comminution efficiency in shock wave lithotripsy. J Urol. 2004. 172:349–354.
5. Pace KT, Ghiculete D, Harju M, Honey RJ. University of Toronto Lithotripsy Associates. Shock wave lithotripsy at 60 or 120 shocks per minute: a randomized, double-blind trial. J Urol. 2005. 174:595–599.
6. Chacko J, Moore M, Sankey N, Chandhoke PS. Does a slower treatment rate impact the efficacy of extracorporeal shock wave lithotripsy for solitary kidney or ureteral stones? J Urol. 2006. 175:1370–1373.
7. Greenstein A, Matzkin H. Does the rate of extracorporeal shock wave delivery affect stone fragmentation? Urology. 1999. 54:430–432.
8. Yilmaz E, Batislam E, Basar M, Tuglu D, Mert C, Basar H. Optimal frequency in extracorporeal shock wave lithotripsy: prospective randomized study. Urology. 2005. 66:1160–1164.
9. Willis LR, Evan AP, Connors BA, Handa RK, Blomgren PM, Lingeman JE. Prevention of lithotripsy-induced renal injury by pretreating kidneys with low-energy shock waves. J Am Soc Nephrol. 2006. 17:663–673.
10. Csáthy L, Pócsi I. Urinary N-acetyl-beta-D-glucosaminidase determination in newborns and children: methods and diagnostic applications. Eur J Clin Chem Clin Biochem. 1995. 33:575–587.
11. Skalova S, Chladek J. Urinary N-acetyl-beta-D-glucosaminidase activity in healthy children. Nephrology (Carlton). 2004. 9:19–21.
12. Turecky L, Uhlikova E. Diagnostic significance of urinary enzymes in nephrology. Bratisl Lek Listy. 2003. 104:27–31.
13. Kim SW, Sul CK. Evaluation of renal injury in patients with renal stone after ESWL. Korean J Urol. 1996. 37:325–330.
14. Koo V, Beattie I, Young M. Improved cost-effectiveness and efficiency with a slower shockwave delivery rate. BJU Int. 2010. 105:692–696.
15. Kim JH, Moon YT. Predicting the therapeutic effect of extracorporeal shockwave lithotripsy by non-enhanced computed tomography in renal stones. Korean J Urol. 2008. 49:252–256.
16. Semins MJ, Trock BJ, Matlaga BR. The effect of shock wave rate on the outcome of shock wave lithotripsy: a meta-analysis. J Urol. 2008. 179:194–197.
17. Talic RF, Rabah DM. Effect of modification of shock-wave delivery on stone fragmentation. Curr Opin Urol. 2006. 16:83–87.
18. Zeman RK, Davros WJ, Garra BS, Horii SC. Cavitation effects during lithotripsy. Part I. Results of in vitro experiments. Radiology. 1990. 177:157–161.
19. Choi MJ, Coleman AJ, Saunders JE. The influence of fluid properties and pulse amplitude on bubble dynamics in the field of a shock wave lithotripter. Phys Med Biol. 1993. 38:1561–1573.
20. Karlsen SJ, Smevik B, Hovig T. Acute morphological changes in canine kidneys after exposure to extracorporeal shock waves. A light and electron microscopic study. Urol Res. 1991. 19:105–115.
21. Recker F, Hofmann W, Bex A, Tscholl R. Quantitative determination of urinary marker proteins: a model to detect intrarenal bioeffects after extracorporeal lithotripsy. J Urol. 1992. 148:1000–1006.
22. Delvecchio F, Auge BK, Munver R, Brown SA, Brizuela R, Zhong P, et al. Shock wave lithotripsy causes ipsilateral renal injury remote from the focal point: the role of regional vasoconstriction. J Urol. 2003. 169:1526–1529.
23. Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000. 11:310–318.
24. Karlin GS, Schulsinger D, Urivetsky M, Smith AD. Absence of persisting parenchymal damage after extracorporeal shock wave lithotripsy as judged by excretion of renal tubular enzymes. J Urol. 1990. 144:13–14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download