Abstract
Purpose
The survival benefits of adjuvant androgen-deprivation therapy (ADT) in prostate cancer and lymph node metastasis remain unclear. We assessed the role of ADT in disease progression after radical prostatectomy (RP).
Materials and Methods
Of 937 patients who underwent RP, we identified 40 (4.2%) who had lymph node metastasis. A total of 18 received adjuvant ADT (ADT group) and 22 were observed (observation group). Clinical progression-free survival (PFS), cancer- specific survival (CSS), and overall survival (OS) were compared in the 2 groups. Prognostic factors for clinical progression and biochemical recurrence (BCR) were analyzed.
Results
The 5-year PFS, CSS, and OS of the entire cohort were 75.0%, 85.0%, and 72.5%, respectively. In the ADT group, 6 patients (33.3%) showed clinical progression at a median 42.7 months. The 5-year PFS, CSS, and OS rates of this group were 72.2%, 83.3%, and 72.2%, respectively. In the observation group, 14 patients (63.6%) received salvage therapy owing to BCR. Nine patients (40.9%) with BCR in the observation group showed clinical progression at a median 43.4 months after RP. The 5-year PFS, CSS, and OS rates of this group were 77.2%, 86.4%, and 72.8%, respectively. In the observation group, the BCR rate was lower in patients with pT3a or less disease than in those with pT3b disease.
Conclusions
Adjuvant ADT in node-positive prostate cancer did not reduce or delay disease progression or improve survival. Because a substantial number of untreated patients with pT3a or less disease did not experience recurrence, administration of ADT should be initiated carefully. However, in patients with pT3b disease, adjuvant ADT and radiotherapy could be considered.
Prostate-specific antigen (PSA) screening has resulted in a down-staging of prostate cancer [1], with the incidence of nodal metastases decreasing from 20% to 40% in the 1980s to 5% to 10% more recently [2,3]. The extent of lymph node dissection and the patient selection criteria might be other causes of these differences [4,5]. Lymph node metastasis is generally regarded to be a poor prognostic indicator in patients with prostate cancer [6,7]. In patients with nodal metastases, nodal progression develops at a median of 18 to 24 months after radical prostatectomy (RP) [2], with a 5 year overall survival (OS) rate of 39.5% [8]. Although androgen-deprivation therapy (ADT) has been shown to benefit patients with high-risk, localized disease who are undergoing radiotherapy [9-11], its role in patients with nodal metastases after RP is unclear. Although immediate adjuvant ADT in patients with nodal metastasis after RP has been found to improve OS and progression-free survival (PFS) [10,12], controversy remains about the treatment effect of adjuvant ADT on survival. Furthermore, the appropriate timing and duration of adjuvant ADT remain to be clarified.
In addition, because long-term ADT has the potential for serious side effects, including osteoporosis, cardiovascular disease, diabetes, and mood disorder [13], the initiation and maintenance of ADT should be considered carefully. We therefore assessed the role of adjuvant ADT for 2 years on disease progression after RP for node-positive prostate cancer.
Of 937 patients who underwent RP performed by a single surgeon between 1990 and 2008, we identified 40 (4.2%) who had lymph node metastasis. Of these 40 patients, 18 received adjuvant ADT for 2 years (ADT group), whereas the remaining 22 were followed without therapy (observation group). Forms of ADT included bilateral orchiectomy in 2 patients and maximal androgen blockade including LHRH agonist injection and oral antiandrogens in the remaining 16 patients.
Postoperatively, PSA was measured every 3 months for the first 2 years, and every 6 months thereafter, unless recurrence was suspected. In all patients, PSA was undetectable at the first hospital visit except in 3 patients in the observation group with PSA levels of 13.6 ng/ml, 23.4 ng/ml, and 20.6 ng/ml. Radiographic evaluation included abdominopelvis computed tomography (CT) or pelvic magnetic resonance (MR) and radionuclide bone scans, which were performed every 6 months and when clinically indicated. Biochemical recurrence (BCR) after RP was defined as two consecutive serum PSA concentrations >0.2 ng/ml, and clinical progression was defined as local recurrence or distant metastasis on imaging study. After clinical progression was detected, two patients (2/6) in the ADT group and three patients (3/9) in the observation group received radiotherapy.
Clinicopathological factors and 5 year PFS, cancer-specific survival (CSS), and OS rates were compared in the two groups by using chi-squared tests, Kaplan-Meier analysis, and the log rank test. SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses, with a p-value ≤0.05 considered statistically significant. In the ADT group, predictive factors of clinical progression were evaluated; in the observation group, the incidence and predictive factors of BCR were analyzed. None of the patients in the ADT group experienced severe complications requiring discontinuation of therapy. The median length of follow- up after surgery was 55.7 months.
At the time of RP, the mean patient age was 64.0 years and the mean serum PSA concentration was 34.8 ng/ml. Of the 40 patients, 31 (77.5%) had surgical Gleason scores ≥8 and 27 (67.5%) had tumors of pathological stage T3b. There were no significant differences between the ADT group and the observation group in age, preoperative PSA, surgical Gleason score, pathological stage, number of positive lymph nodes, or percentage with positive surgical margins (Table 1).
The clinical 5 year PFS, CSS, and OS rates of the entire patient cohort were 75.0%, 85.0%, and 72.5%, respectively. Six (33%) patients in the ADT group experienced clinical progression at a median 42.7 months. In this group, five patients died, three due to prostate cancer, making the 5 year PFS, CSS, and OS rates of this group 72.2%, 83.3%, and 72.2%, respectively. In the observation group, 14 (63.6%) patients received salvage hormonal therapy owing to BCR, which was observed at a median 22.9 months after RP. The remaining eight (36.4%) patients did not experience BCR during a median follow-up of 32.3 months. Nine patients (9/22, 40.9%) in the observation group experienced clinical progression at a median 43.4 months after RP and a median 20.5 months after salvage hormonal therapy. In this group, six patients died, three due to prostate cancer, making the 5 year PFS, CSS, and OS rates 77.2%, 86.4%, and 72.8%, respectively. There were no significant differences between the groups in clinical progression or survival (Figs. 1, 2). The pattern of clinical progression in the ADT group included three patients with bone metastases, two with lymph node metastases, and one with metastasis at the local prostatectomy site; the mean PSA level of these patients was 19.1 ng/ml (range, 1.1 to 81.0 ng/ml). In the observation group, five patients had bone metastases, three had lymph node metastases, and one had lung metastasis; the mean PSA level was 42.7 ng/ml (range, 0.04 to 341 ng/ml).
Age, preoperative PSA, surgical Gleason score, number of positive lymph nodes, and positive surgical margin were not prognostic for clinical progression in the ADT group or for BCR in the observation group. In the observation group, however, BCR developed in 12 of 13 patients (92.3%) with pT3b tumors and in 2 of 9 patients (22.2%) pT3a or less (Table 2). Of the 14 patients with pT3b disease in the ADT group, 6 had clinical progression at a median 42.2 months after RP. Of 13 patients in the observation group with pT3b disease, 12 received salvage hormonal therapy due to BCR at a median 20.6 months after RP and 6 had clinical progression at a median 22.5 months after salvage therapy.
The benefits of adjuvant ADT for patients with node-positive prostate cancer after RP are still unclear. We found that 6 of 18 (33.3%) ADT-treated patients experienced clinical progression at a median 42.7 months, compared with 9 of 22 (40.9%) patients in the observation group, who experienced clinical progression at a median 43.4 months, indicating that adjuvant ADT in patients with node-positive prostate cancer did not reduce or delay disease progression. Moreover, there were no between-group differences in the 5 year CSS and OS rates. Our results could be supported by other studies [2]. In contrast, Messing et al reported that immediate adjuvant ADT improved PFS, CSS, and OS in 47 patients who received immediate ADT after RP and in 51 patients who were observed, with ADT to be started after detection of distant metastases or symptomatic recurrences [10]. The discrepancy between these studies may be due to the clinicopathological characteristics of the patients included in the retrospective analyses. That is, although the percentages of patients with pT3b disease and positive surgical margins did not differ, 77.5% of our patients had a Gleason score ≥8 compared with 12.2% in the previous study. Thus, our patients were at higher risk of recurrence although they received ADT, which may have resulted in more developed clinical progression than in the previous study. In addition, the timing and duration of ADT were not mentioned in the previous study [9], whereas we initiated ADT at a mean 4.2 months after RP and continued treatment for 2 years. These differences in timing and duration of ADT may be associated with disease progression.
Of the 22 patients in the observation group, 8 (36.3%) did not develop BCR during a median follow-up of 32.3 months. Although nodal metastases after RP represent a poor pathologic characteristic, not all patients are at the same risk of BCR and cancer-specific death [14,15]. The proportion of patients without any adjuvant therapy not experiencing BCR was reported to be 15% in a previous study [16]. In the current study, we were unable to identify differentiating prognostic factors between the patients developing BCR and those who did not, other than the pathological T stage.
Because BCR did not develop in 36.4% of patients in the observation group and patients with pT3a disease or less had a significantly lower risk of BCR, stage pT3a or less may indicate observation rather than initiation of ADT, thus reducing the adverse effects of long-term ADT. In regard to the initiation of adjuvant ADT in patients with pT3bN+ disease after RP, we found no significant difference in clinical progression between patients with pT3b disease who received adjuvant ADT or salvage hormonal therapy. In patients with pT2-4 prostate cancer, including 64.3% with pT3b disease, and lymph node metastasis, the combination of adjuvant ADT and radiotherapy improved CSS and OS [17]. Similarly, adjuvant ADT plus radiotherapy in patients with node-positive prostate cancer, 60.8% with pT3b disease, improved recurrence-free survival and CSS [18]. In patients with pT3bN+, the combination of adjuvant ADT and radiotherapy may improve disease control compared with adjuvant ADT alone. Because adjuvant ADT failed to demonstrate survival differences or delay clinical progression, the timing of salvage ADT may also be questioned. In failing patients, hormonal manipulation did not seem to extinguish the cancer in the entire population. In this regard, ADT could be reserved until a measurable lesion is formed or clinical symptoms ensue.
This study had several limitations, including its retrospective design and inclusion of a relatively small number of patients with node-positive prostate cancer, thus reducing the power of statistical analysis. A prospective randomized study based on a larger population is necessary. Moreover, multivariate analysis of factors affecting BCR in the observation group could not be done because of the small sample size. The effect of duration of adjuvant ADT may have affected disease progression and patient survival. Therefore, additional studies are needed to clarify the effects of long-term ADT.
Adjuvant ADT in patients with node-positive prostate cancer did not reduce or delay disease progression or improve survival. Because a substantial number of untreated patients with pT3a or less did not develop recurrence after RP, administration of ADT should be initiated carefully in these patients. However, in patients with pT3b disease, adjuvant ADT and radiotherapy could be considered.
Figures and Tables
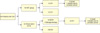 | FIG. 1Clinical course of the patients. LN: lymph node, ADT: androgen deprivation therapy, CP: clinical progression, BCR: biochemical recurrence. |
References
1. Ghavamian R, Bergstralh EJ, Blute ML, Slezak J, Zincke H. Radical retropubic prostatectomy plus orchiectomy versus orchiectomy alone for pTxN+ prostate cancer: a matched comparison. J Urol. 1999. 161:1223–1227.
2. Spiess PE, Lee AK, Busby JE, Jordan JJ, Hernandez M, Burt K, et al. Surgically managed lymph node-positive prostate cancer: does delaying hormonal therapy worsen the outcome? BJU Int. 2007. 99:321–325.
3. Bader P, Burkhard FC, Markwalder R, Studer UE. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol. 2003. 169:849–854.
4. Kroepfl D, Loewen H, Roggenbuck U, Musch M, Klevecka V. Disease progression and survival in patients with prostate carcinoma and positive lymph nodes after radical retropubic prostatectomy. BJU Int. 2006. 97:985–991.
5. Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol. 2002. 167:1681–1686.
6. Zwergel U, Lehmann J, Wullich B, Schreier U, Remberger K, Zwergel T, et al. Lymph node positive prostate cancer: long-term survival data after radical prostatectomy. J Urol. 2004. 171:1128–1131.
7. Daneshmand S, Quek ML, Stein JP, Lieskovsky G, Cai J, Pinski J, et al. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J Urol. 2004. 172:2252–2255.
8. Kramer SA, Cline WA Jr, Farnham R, Carson CC, Cox EB, Hinshaw W, et al. Prognosis of patients with stage D1 prostatic adenocarcinoma. J Urol. 1981. 125:817–819.
9. Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997. 337:295–300.
10. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006. 7:472–479.
11. You D, Jeoung IG, Kim CS. Role of radical prostatectomy for high-risk prostate cancer. Korean J Urol. 2010. 51:589–595.
12. Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999. 341:1781–1788.
13. Wong YN, Freedland S, Egleston B, Hudes G, Schwartz JS, Armstrong K. Role of androgen deprivation therapy for node-positive prostate cancer. J Clin Oncol. 2009. 27:100–105.
14. Cheng L, Zincke H, Blute ML, Bergstralh EJ, Scherer B, Bostwick DG. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer. 2001. 91:66–73.
15. Boorjian SA, Thompson RH, Siddiqui S, Bagniewski S, Bergstralh EJ, Karnes RJ, et al. Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol. 2007. 178:864–870.
16. Gjertson CK, Asher KP, Sclar JD, Goluboff ET, Olsson CA, Benson MC, et al. Local control and long-term disease-free survival for stage D1 (T2-T4N1-N2M0) prostate cancer after radical prostatectomy in the PSA era. Urology. 2007. 70:723–727.
17. Briganti A, Karnes RJ, Da Pozzo LF, Cozzarini C, Capitanio U, Gallina A, et al. Combination of adjuvant hormonal and radiation therapy significantly prolongs survival of patients with pT2-4 pN+ prostate cancer: results of a matched analysis. Eur Urol. 2011. 59:832–840.
18. Da Pozzo LF, Cozzarini C, Briganti A, Suardi N, Salonia A, Bertini R, et al. Long-term follow-up of patients with prostate cancer and nodal metastases treated by pelvic lymphadenectomy and radical prostatectomy: the positive impact of adjuvant radiotherapy. Eur Urol. 2009. 55:1003–1011.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download