Abstract
A 75-year-old female visited our hospital with bilateral adrenal masses that were detected incidentally during lumbar spine magnetic resonance imaging (MRI) for the evaluation of radiating flank pain. Consecutive computed tomography and MRI revealed bilateral adrenal masses with no evidence of lymph node enlargement or local invasion; 2[(18)F]fluoro-2-deoxyglucose (FDG)-positron emission tomography showed an intense FDG accumulation in both adrenal glands without abnormal FDG uptake in extra-adrenal regions. The laboratory test results were within normal ranges. We performed a bilateral adrenalectomy. The pathologic diagnosis of both adrenal masses was consistent with adrenocortical carcinoma. The patient recovered well with no complications.
Primary malignant tumors originating from the adrenal gland include adrenocortical carcinomas, primary adrenal lymphomas, and malignant pheochromocytomas; however, the incidence of these tumors is low [1-4]. Most adrenal tumors are sporadic and unilateral, but 2% to 6% of adrenal tumors are bilateral and associated with Li-Fraumeni syndrome, type I multiple endocrine neoplasia, Beckwith-Wiedemann syndrome, and Carney complex, principally in children [1-4]. When bilateral adrenal masses are detected, an effort is undertaken to find other primary malignant foci.
There are a few reported cases of bilateral primary adrenocortical carcinomas [1]. Here we report one such case that was successfully managed with a bilateral adrenalectomy and discuss the relevant literature.
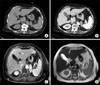
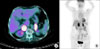
A 75-year-old female was referred to our clinic with bilateral adrenal masses that were detected incidentally by lumbar spine magnetic resonance imaging (MRI) for evaluation of radiating flank pain. She presented with a high blood glucose level that had been controlled with medical treatment for 10 years. She had undergone surgery for a compressive fracture of the lumbar spine 3 months previously. A contrast-enhanced computer tomography (CT) scan was performed and revealed a left adrenal mass with inhomogeneous enhancement after application of contrast medium (40×18 mm) (Fig. 1A and 1B). On axial MRI, the bilateral adrenal masses had high-signal intensity on T1- and T2-weighted images and a heterogeneous enhancement pattern (left, 48×19 mm; right, 29×23 mm) (Fig. 1C and 1D). There was no evidence of lymph node enlargement or local invasion. For evaluation of other malignant lesion or metastases, a 2[(18)F]fluoro-2-deoxyglucose (FDG)-positron emission tomography (PET) scan was performed and showed an intense FDG accumulation in the bilateral adrenal masses without abnormal FDG uptake in extra-adrenal regions (left, 38×20 mm; right, 35×28 mm) (Fig. 2A). The results of adrenal function tests were within normal ranges. We performed a bilateral adrenalectomy via a bilateral subcostal approach. The pathologic evaluation confirmed the diagnosis of adrenocortical carcinomas. The macroscopic findings were as follows: left adrenal tumor, 16×14 mm; and right adrenal tumor, 39×15 mm. The adrenal masses reveal marked nuclear pleomorphism with compact eosinophilic cytoplasm, numerous mitoses, and necrosis. The adrenal masses were graded according to the Weiss criteria (0-9) with a score of 5 microscopically (Fig. 3). The patient recovered well with no complications at the 3-month follow-up.
Neoplastic involvement of the adrenal gland may result from primary tumors originating from the adrenal cortex of the adrenal medulla. Primary malignant tumors originating from the adrenal gland include adrenocortical carcinomas, primary adrenal lymphomas, and malignant pheochromocytomas. Adrenal glands are more frequently the site of metastatic disease caused by primary carcinomas. Any primary cancer can spread to the adrenal glands; lymphomas, lung cancer, melanomas, leukemia, renal carcinoma, and ovarian carcinoma account for the majority of adrenal metastases [1,4].
The incidence of adrenocortical carcinoma is estimated to be 0.4/100,000. Adrenocortical carcinoma increases with tumor size to 25/100,000 (median diameter >6 cm) [4]. Bilateral manifestations of adrenocortical carcinoma occur in only 10% of the cases reported [1].
In contrast to our patient, many patients with adrenocortical carcinomas present with clinical symptoms of endocrine excess. Indeed, hormone-functioning tumors account for 26% to 94% of adrenocortical carcinomas [3,4]. Most patients with adrenocortical carcinomas are diagnosed at an advanced stage of disease with large primary tumors (median tumor size at diagnosis, >10 cm) and invasion to adjacent organs. The main clinical symptoms, such as abdominal discomfort or back pain, are related to the mass effect of a large tumor [3,4].
All adrenal tumors detected have to be diagnosed for malignancy potential and hormonal activity to render timely and curative treatment. Imaging studies using CT, MRI, and FDG-PET to demonstrate adrenal mass size and appearance have been used to distinguish between benign and malignant lesions. Differentiation between malignant and benign adrenal lesions can be performed by using 18-FDG-PET with >95% accuracy [4,5]. In particular, 18-FDG-PET plays an important role in evaluating treatment response and residual masses.
In general, surgery involving adrenal tumors should be considered in patients with functioning cortical tumors and clinical symptoms [4,6,7]. Regarding nonfunctioning tumors, recommendations regarding treatment mainly refer to the tumor size. In general, clinically silent lesions <3 cm without any criteria of malignancy are not resected and should be followed closely by CT or MRI scans every 6 or 12 months [4,6,7]. The indications for an adrenalectomy are a definitive or presumed diagnosis of primary adrenocortical carcinoma and circumstances technically obstructive to a minimally invasive approach. In the case of any intraoperative features of malignancy, conversion to an open approach should be performed to enable extensive radical compartment resection [3,6,7]. In our case, the intraoperative findings suggested a malignancy. Thus, we performed a bilateral adrenalectomy.
The differentiation between benign and malignant adrenal lesions is based on macroscopic and microscopic features [3,8]. The Weiss score is the most widely used classification for microscopic characteristics suggestive of a malignant tumor. Three or more histologic criteria are necessary to establish the diagnosis of adrenal carcinoma [9]. In our case, marked nuclear atypia, frequent mitoses (8-10/10 high power fields), vascular and capsular invasion, and necrosis were found. Therefore, the diagnosis of primary bilateral adrenocortical carcinoma was established.
Figures and Tables
FIG. 1
Preoperative computed tomography and axial magnetic resonance imaging. (A, B) Left adrenal tumor with inhomogeneous enhancement is shown. (C, D) Bilateral adrenal masses have heterogeneously high-signal intensity on both T1- and T2-weighted images.
References
1. Ozimek A, Diebold J, Linke R, Heyn J, Hallfeldt K, Mussack T. Bilateral primary adrenal non-Hodgkin's lymphoma and primary adrenocortical carcinoma-review of the literature preoperative differentiation of adrenal tumors. Endocr J. 2008. 55:625–638.
2. Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol. 2002. 179:559–568.
3. Allolio B, Fassnacht M. Clinical review: Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab. 2006. 91:2027–2037.
4. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004. 25:309–340.
5. Pacak K, Eisenhofer G, Goldstein DS. Functional imaging of endocrine tumors: role of positron emission tomography. Endocr Rev. 2004. 25:568–580.
6. Palazzo FF, Sebag F, Sierra M, Ippolito G, Souteyrand P, Henry JF. Long-term outcome following laparoscopic adrenalectomy for large solid adrenal cortex tumors. World J Surg. 2006. 30:893–898.
7. Valeri A, Bergamini C, Manca G, Mannelli M, Presenti L, Peri A, et al. Adrenal incidentaloma: The influence of a decision-making algorithm on the short-term outcome of laparoscopy. J Laparoendosc Adv Surg Tech A. 2005. 15:451–459.
8. Aiba M, Fujibayashi M. Histopathological diagnosis and prognostic factors in adrenocortical carcinoma. Endocr Pathol. 2005. 16:13–22.
9. Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984. 8:163–169.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download