Abstract
Purpose
Detrusor overactivity (DO) cannot be predicted by clinical symptoms. Although it is possible that DO could be related to anatomical structures, scanty data exist about the relations between DO and anatomical structures. The aim of this study was to investigate anatomical differences in DO by measuring the thickness of the urethrovaginal space (UVS) and the urethral length (UL) in women with stress urinary incontinence (SUI).
Materials and Methods
Prospective data were collected from 72 women with SUI who underwent the midurethral sling operation. The subjects were divided into 2 groups according to the presence of DO by preoperative urodynamic study (UDS). UVS thickness was measured by trans-vaginal ultrasound. UL was measured by using a urethral catheter and a ruler. UVS thickness, UL, Q-tip, and urodynamic parameters, such as maximal urethral closure pressure (MUCP) and Valsalva leak point pressure, were compared between the two groups.
Results
Of 72 women, 23 patients had DO (31.9%). The proximal UVS was significantly thinner (p<0.001) and the MUCP was significantly lower (p=0.008) in women with DO. According to the receiver operating characteristic (ROC) curve based DO prediction, the best cutoff value for UVS thickness was 0.84 cm (area under the ROC curve 0.763).
Conclusions
In this study, the proximal UVS was significantly thinner and the MUCP was significantly lower in patients with DO. A proximal UVS thickness of less than 0.84 cm was shown to be a predictive parameter for the development of DO on preoperative UDS. A large-scale prospective study is needed to validate these results.
Stress urinary incontinence (SUI) is due to the involuntary leakage of urine without bladder contraction secondary to a sudden increase in intra-abdominal pressure. It is the most common cause of urinary incontinence in adult women, in whom the incidence ranges between 40% and 65% [1].
Mixed urinary incontinence (MUI) is defined as involuntary leakage of urine associated with urgency and exertion, effort, sneezing, or coughing [2,3]. Its prevalence tends to increase with age [4]. It has been reported that between 30% and 50% of incontinent women experience MUI [5].
Detrusor overactivity (DO) has been proven through objective evaluation with urodynamic study (UDS), and not all MUI patients exhibit DO on UDS. When urinary incontinence is combined with DO, the patient's quality of life decreases markedly and the efficacy and satisfaction with anti-incontinence surgery decline. We previously reported that women with MUI associated with DO had no differences in demographical and clinical variables before anti-incontinence surgery but experienced less satisfaction after surgery than did those without DO [6].
Even if its causes have not been clearly delineated in SUI patients, leakage of urine occurs when urethral internal pressure becomes less than that of the bladder as intra-abdominal pressures increase from the weakening support structure of the urethra and bladder floor. In a study of the anatomical structures of the female lower urinary tract, Tunn et al demonstrated reduced urethral sphincter muscle thickness, changes of the levator ani muscle, and defects of the endopelvic fascia by performing magnetic resonance imaging (MRI) in women with SUI [7]. Choi et al reported that the urethral length (UL) was significantly shorter and the distal anterior vaginal wall was significantly thicker in women with SUI [8]. Therefore, it appears that the urethra and the tissues around the bladder floor play an important role in the pathogenesis of SUI.
The reason for the lack of reproducibility of DO in SUI is that SUI might also be the primary pathophysiologic mechanism responsible for MUI. In these women, the incompetent urethral sphincter and bladder neck allow urine to enter the proximal urethra during physical activity, eliciting an urethro-detrusor facilitative reflex that triggers involuntary detrusor contraction, causing urgency and urge urinary incontinence (UUI) [9]. Thus, there is a clinical discrepancy with symptomatic MUI and the actual presentation of DO on UDS.
On the other hand, research is lacking on the relations between DO and female anatomical structures. The aim of this study was to investigate whether female anatomical variables such as urethrovaginal space (UVS) thickness and UL are major factors influencing DO in women with SUI.
Prospective data were collected from 72 women who underwent anti-incontinence surgery between March 2009 and March 2011 after approval of the Institutional Review Board of the Korea University Hospital. Investigations were carried out on all the subjects before surgery, which included a medical history, physical examination, symptom questionnaire, urinalysis and urine culture, Q-tip test, uroflowmetry, measurement of post-void residual urine volume, and UDS.
SUI was classified according to the Stamey grade into three categories. MUI was defined as involuntary urinary leakage associated with urgency and exertion, effort, sneezing, or coughing [2]. Patients with significant cystocele, pelvic floor prolapse, history of pelvic surgery, or psycho-neurologic conditions were excluded.
UDS included measurement of Valsalva leak point pressure (VLPP) and the maximal urethral closure pressure (MUCP) according to the guidelines of the International Continence Society [10]. DO was defined as involuntarily increased urination pressure by more than 15 cmH2O or increased with a sense of urinary urgency. Intrinsic sphincter deficiency (ISD) was defined as less than 60 cmH2O of VLPP. The MUCP was measured by inserting a 6 Fr urethral catheter into the bladder through the urethra and then pulling it from the bladder at a rate of 1.0 mm/s while injecting saline at a rate of 2.0 ml/s.
The thickness of the proximal UVS was measured by trans-vaginal ultrasound. After positioning the patient in the lithotomy position and inserting an 18 Fr urethral catheter and inflating it with 10 ml distilled water, an 8 MHz trans-vaginal probe was used to measure the thickness of the proximal UVS. The thickness of the proximal UVS was measured by the distance between the anterior vaginal wall and the catheter balloon, after halting the screen while viewing the vaginal wall and the catheter balloon on trans-vaginal ultrasound (Fig. 1). In addition, UL was measured as follows: first, the catheter balloon was pulled to the maximum to place it on the bladder neck; second, the gradation was marked on the part related to the urethral meatus; third, the catheter was removed by removing the balloon; fourth, the balloon was re-inflated outside the body. Finally, the UL was measured as the distance from the near side of the balloon to the urethral meatus with a ruler (Fig. 2).
The subjects were divided into 2 groups according to DO status. UVS, UL, Q-tip, and urodynamic parameters such as VLPP and MUCP were compared between the two groups.
The Fisher's exact test and the Mann-Whitney U test were used to compare the two groups. The strength of association between parameters was assessed by using the Spearman correlation analysis. Receiver operating characteristic (ROC) curves were calculated to set cutoff values for each parameter. SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analyses, and p-values less than 0.05 (p<0.05) were considered statistically significant.
The clinical characteristics of the 72 patients with SUI are summarized in Table 1 and Table 2. The mean patient age was 56.7 years (range, 32 to 76 years). Of the 72 women, 23 had DO (31.9%) by preoperative UDS and 49 patients did not (68.1%). Of the women with MUI, 31.4% had shown DO in the preoperative UDS. Urodynamic diagnosis of DO was predicted only by the existence of urgency symptoms (p=0.083). Other factors such as MUI or ISD were not meaningfully related with DO (Table 1).
The median age of the women with DO was 58.0 years and that of the women without DO was 53.0 years. The median symptom duration of women with DO was 10.0 months and that of women without DO was 7.0 months. The median parity was 2.0 in women with and without DO. There were no significant differences in age, symptom duration, SUI grade, urodynamic parameters except MUCP, voiding diary, or uroflowmetry between the two groups (Table 2).
The proximal UVS was significantly thinner in women with DO than in women without DO (p<0.001). The UL of women with DO was shorter than that of women without DO, albeit without significance (p=0.357). MUCP was significantly lower in women with DO than in women without DO (p=0.008).
Anatomical variables and variables that were statistically significant in the univariate analysis, such as proximal UVS, UL, and MUCP, were analyzed by using the ROC curve. The area under the curve (AUC) of each factor was 0.763, 0.567, and 0.694, respectively (Fig. 3). The AUC of proximal UVS and MUCP showed significant differences. At the cutoff value of 0.84 cm for proximal UVS, the sensitivity and specificity were 95.7% and 81.6%, respectively.
Correlation coefficients between the preoperative parameters were obtained (Table 3). An intermediate negative correlation (correlation coefficient -0.474) was found between MUCP and age (p<0.001).
MUI is defined as the state of SUI combined with UUI [3]. It is an unclear term because it can apply to a combination of incontinent symptoms (SUI and UUI) and to a combination of urodynamic conditions (urodynamic SUI and DO) in the same individual. Nevertheless, it is generally taken to represent symptoms. UDS is a useful method for studying women with MUI to determine the underlying pathology, particularly if the patients have not responded to conservative treatment. However, it is well known that up to 50% of patients with UUI do not demonstrate DO on UDS, just as not all women with SUI show leakage during UDS [11]. The reason is that with the urethro-detrusor facilitative reflex, SUI might also be the primary pathophysiologic mechanism responsible for MUI [9]. Also, in our study, there was a clinical discrepancy with symptomatic MUI and the actual presentation of DO on UDS.
It is generally known that 50% to 74% of patients with MUI can expect recovery of complete urinary continence because the factors of urinary urgency can be completely reversed by anti-incontinence surgery [12]. However, it has also been reported that patients with MUI experience a lower complete cure rate and satisfaction after surgery than do those with SUI only, with a rate of 36% to 66% [13]. It has also been reported that DO persisted after surgery in 4 of 12 patients with DO, and SUI persisted after surgery in 9 patients, whereas urinary incontinence resolved after surgery in 32 of 50 patients [13]. In short, a main risk factor for surgical failure for MUI is the existence of DO before surgery [14].
It has been reported that UVS thickness is related to sexual excitement [15,16]. A study that entailed trans-vaginal ultrasonography was used to measure the thickness of the anterior vaginal wall to investigate the anatomical variability of a clitoris-UVS complex called the G-spot [17]. UVS can be correlated with complications after surgery, such as bladder irritability and tissue damage at the time of inserting the mesh required for sling surgery. Studies have been conducted on the thickness of the anterior vaginal wall in patients with SUI. It is assumed that the anterior vaginal wall around the distal urethra thickens as the distal urethral muscle becomes more involved with the closure of the urethra owing to a compensatory mechanism in patients with urinary incontinence, who have a more opened proximal urethral meatus related to the damage to the comparative rhabdoid urethral muscle and the volume loss of the connective tissue. However, accurate evidence is still lacking, even if the thickness of the UVS is assumed to be a factor that exerts influence on the pathogenesis of MUI. In this study, we found that the proximal UVS was significantly thinner in patients with DO. This suggests that the thinner the proximal UVS, the higher the possibility that ISD may be involved. Urinary leakage from the posterior urethra in SUI due to ISD stimulates the urethral sensory nerves to promote a voiding reflex that may then cause DO [9].
Considering the studies related to hormonal changes such as estrogen secondary to an increase in age and UVS thickness, it appears that the incidence of atrophic vaginitis increases due to a reduction in estrogen secretion due to menopause, and it can be a cause of reducing UVS thickness [18,19]. In addition, considering that symptoms of urinary urgency or UUI occurring after menopause were improved by estrogen replacement therapy [20], DO symptoms such as urinary urgency and UUI can occur due to a decrease in circulating estrogen secondary to menopause. At the same time, it can be considered that DO will occur more frequently in patients with a thinner UVS as the UVS becomes thinner as the result of atrophic vaginitis. However, a large-scale systematic study of this hypothesis should be conducted in the future.
A short UL has been reported to be a factor for SUI even if the correlation between UL and urinary incontinence is open to dispute [8]. Choi et al and Koo and Lee concluded that the length of a functional urethra in patients with urinary incontinence shown by UDS was significantly shorter than that of women without urinary incontinence [8,20]. On the other hand, there was a report that the rhabdoid urethral muscle was found to be substituted with hyper-dense tissue in patients with serious SUI through perineum ultrasonography [21], and Kondo et al asserted that a correlation between SUI grade and volume of the rhabdoid urethral muscle existed [22]. To sum up all these findings, it can be assumed that the UL is shorter in patients with SUI than in those without, owing to a reduction in the volume of the rhabdoid urethral muscle and weakening of the support structures around the bladder neck and urethra. However, research is scarce on UL in patients with MUI. The present study measured and compared the UL of patients with DO and that of patients without DO among SUI patients. Even if UL was shorter in patients with DO than in those without, there was no statistically significant difference between them. In addition, it has been shown that UL is negatively correlated with symptom duration.
This study demonstrated that the MUCP was significantly lower in patients with DO than in those without. The results of this study are consistent with those of an earlier study that reported that the rhabdoid urethral muscle was thinner and the MUCP was lower in patients with DO [23]. Awad et al reported that the urethral pressure profile in patients with DO was distinctively low on MUCP [24]. It was also mentioned that low MUCP was a factor predicting persistent urinary urgency following sling surgery in patients with MUI [25]. However, these findings are in contrast with the results of Woo et al [26]. Those authors stated that the bladder wall would become thicker when measured by ultrasonography and the detrusor pressure at maximal urine flow rate (PdetQmax) and MUCP were higher and the length of a functional urethra was longer in patients with DO than in normal individuals. This appears to be related to the increase in bladder outlet resistance caused by hypertrophy of the urethral sphincter and increased tension levels. MUCP was negatively correlated with age and positively correlated with PdetQmax and maximal detrusor pressure (Pdetmax) in this study. Because studies have generated different results on the correlation between the MUCP and DO, additional research is needed on the implications and correlations of these results.
There are several limitations to our study. First, a relatively small number of patients render it challenging to define the exact role of UVS and UL. Second, only one investigator conducted and interpreted the measurement of UVS thickness and the measurement of UL to reduce inter-individual errors. However, proximal UVS thickness (cm) showed a 2 mm difference for the average value even though there was statistical difference between the group with DO (0.5±0.2) and the group without DO (0.7±0.2). This error can occur according to what degree the experimenter pulls the urethral catheter. The methods of measuring UVS and UL should be validated by another method, such as MRI, but in clinical practice it is not easy to perform MRI in SUI patients.
The objective of this study was to characterize MUI in relation to DO by using UVS and UL by means of a relatively simple method. Large-scale prospective studies are needed to determine the critical role of UVS and UL in patients with DO.
Proximal UVS was significantly thinner and MUCP was significantly lower in women with SUI with DO than in women with SUI without DO. To achieve good results and patient satisfaction after anti-incontinence surgery, it is imperative to consider the anatomical differences between patients with SUI preoperatively. Large-scale prospective studies are needed to establish the potential role of UVS and UL together with other anatomical structures.
Figures and Tables
FIG. 1
Measurement of proximal urethrovaginal space by transvaginal ultrasonography (the arrow represents the distance between the anterior vaginal wall and the catheter balloon).
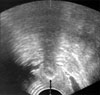
FIG. 2
Measurement of urethral length by use of a Foley catheter (the mark on the catheter represents the point of the urethral meatus and the proximal end of the balloon represents the point of the bladder neck, respectively).

FIG. 3
Receiver operating characteristic (ROC) curve of urethral length (UL), proximal urethrovaginal space (UVS), and maximal urethral closure pressure (MUCP) for detrusor overactivity (AUC: 0.567, 0.763, and 0.694, respectively).

TABLE 2
Baseline demographic and clinical data of women with stress urinary incontinence according to the presence or absence of detrusor overactivity by preoperative urodynamic study

Data are mean value±SD with median value or number of patients (%). UVS: urethrovaginal space, SUI: stress urinary incontinence, VLPP: Valsalva leak point pressure, MUCP: maximal urethral closure pressure, FUL: functional urethral length, PdetQmax: detrusor pressure at maximal urine flow rate, Pdetmax: maximal detrusor pressure, VV: voided volume, RV: residual urine volume. a: p<0.05 by Mann-Whitney U test
Notes
References
1. Seo JB, Lee JZ. The epidemiologic study of the urinary incontinence in community-dwelling women over 50 years old. Korean J Urol. 1999. 40:1525–1530.
2. Brubaker L, Stoddard A, Richter H, Zimmern P, Moalli P, Kraus SR, et al. Mixed incontinence: comparing definitions in women having stress incontinence surgery. Neurourol Urodyn. 2009. 28:268–273.
3. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the international continence society. Neurourol Urodyn. 2002. 21:167–178.
4. Simeonova Z, Milsom I, Kullendorff AM, Molander U, Bengtsson C. The prevalence of urinary incontinence and its influence on the quality of life in women from an urban Swedish population. Acta Obstet Gynecol Scand. 1999. 78:546–551.
5. Dmochowski R, Staskin D. Mixed incontinence: definitions, outcomes, and interventions. Curr Opin Urol. 2005. 15:374–379.
6. Kim JJ, Bae JH, Lee JG. Preoperative factors predicting the outcome of a midurethal sling operation for treating women with mixed incontinence. Korean J Urol. 2008. 49:1112–1118.
7. Tunn R, Goldammer K, Neymeyer J, Gauruder-Burmester A, Hamm B, Beyersdorff D. MRI morphology of the levator ani muscle, endopelvic fascia, and urethra in women with stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2006. 126:239–245.
8. Choi JD, Koh SB, Kim DY. Comparison of urethral length and anterior vaginal wall thickness between continent and incontinent women. Korean J Urol. 2009. 50:28–32.
9. Jung SY, Fraser MO, Ozawa H, Yokoyama O, Yoshiyama M, De Groat WC, et al. Urethral afferent nerve activity affects the micturition reflex; Implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999. 162:204–212.
10. Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002. 21:261–274.
11. Chaliha C, Khullar V. Mixed incontinence. Urology. 2004. 63:3 Suppl 1. 51–57.
12. Anger JT, Rodríguez LV. Mixed incontinence: stressing about urge. Curr Urol Rep. 2004. 5:427–431.
13. Yun HC, Lee JG. Is urodynamic evaluation necessary for women with stress urinary incontinence? Korean J Urol. 2002. 43:687–692.
14. Bates CP, Loose H, Stanton SL. The objective study of incontinence after repair operations. Surg Gynecol Obstet. 1973. 136:17–22.
15. Tunuguntla HS, Gousse AE. Female sexual dysfunction following vaginal surgery: a review. J Urol. 2006. 175:439–446.
16. Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC. Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002. 99:281–289.
17. Gravina GL, Brandetti F, Martini P, Carosa E, Di Stasi SM, Morano S, et al. Measurement of the thickness of the urethrovaginal space in women with or without vaginal orgasm. J Sex Med. 2008. 5:610–618.
18. Ibe C, Simon JA. Vulvovaginal atrophy: current and future therapies (CME). J Sex Med. 2010. 7:1042–1050.
19. Lynch C. Vaginal estrogen therapy for the treatment of atrophic vaginitis. J Womens Health (Larchmt). 2009. 18:1595–1606.
20. Koo JM, Lee TY. Characteristic changes of cough urethral pressure profile in stress urinary incontinence. Korean J Urol. 1994. 35:665–670.
21. Digesu GA, Robinson D, Cardozo L, Khullar V. Three-dimensional ultrasound of the urethral sphincter predicts continence surgery outcome. Neurourol Urodyn. 2009. 28:90–94.
22. Kondo Y, Homma Y, Takahashi S, Kitamura T, Kawabe K. Transvaginal ultrasound of urethral sphincter at the mid urethra in continent and incontinent women. J Urol. 2001. 165:149–152.
23. Major H, Culligan P, Heit M. Urethral sphincter morphology in women with detrusor instability. Obstet Gynecol. 2002. 99:63–68.
24. Awad S, McGinnis R. Factors that influence the incidence of detrusor instability in women. J Urol. 1983. 130:114–115.
25. Paick JS, Ku JH, Kim SW, Oh SJ, Son H, Shin JW. Tension-free vaginal tape procedure for the treatment of mixed urinary incontinence: significance of maximal urethral closure pressure. J Urol. 2004. 172:1001–1005.
26. Woo JH, Hong SJ, Lee JB. The relationship of pressure-flow parameters and urethral pressure in female patients with lower urinary tract symptoms. Korean J Urol. 2009. 50:567–572.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download