Abstract
Purpose
Sexual adverse events (AEs), a major cause for discontinuing 5α-reductase inhibitor (5ARI) therapy for benign prostatic hyperplasia (BPH), are known to occur most frequently early in therapy and appear to decline over time. The aim of this study was to investigate the changes in sexual function occurring with dutasteride treatment during a 1-year follow-up period in Korean men.
Materials and Methods
Using the International Index of Erectile Function, we prospectively evaluated, after 1, 3, 6, 9, and 12 months of treatment, the changes in sexual function of 55 outpatients (mean age 62.3±7.2 years) with BPH (mean volume 48.9±16.0 g) who had relatively good erectile function (EF) and were treated with dutasteride for at least 1 year.
Results
EF scores showed the most significant decrease at 1 month (p<0.01). Function gradually recovered thereafter but was still significantly decreased after 12 months of treatment (p<0.05). The scores for orgasmic function and sexual desire also showed the most significant reduction at 1 month but were restored to the baseline level at 6 months. No significant correlation was observed between changes in sexual function and prostate-specific antigen level, prostate volume, or International Prostate Symptom Scores.
Conclusions
After 1 month of treatment, dutasteride therapy resulted in a significant reduction in all investigated sexual functions. Overall, recovery in sexual function was noted at 3 months, and orgasmic function and sexual desire were restored to baseline levels at 6 months. However, EF was still significantly reduced at 12 months.
The modern lifestyle and continuously growing aging population in Korea have driven an increase in the prevalence of benign prostatic hyperplasia (BPH) [1], which impairs men's quality of life with lower urinary tract symptoms (LUTS). For symptomatic BPH, medical therapy can be largely categorized into α-blockers and 5α-reductase inhibitors (5ARIs). The α1 type of receptor is predominant in the prostate. Therefore, α1 receptor blockers reduce the functional obstruction of the prostate gland [2,3]. 5ARI inhibits the conversion of testosterone to 5α-dihydrotestosterone (DHT), which is primarily responsible for the initial development and subsequent enlargement of the prostate gland [4]. Thus, 5ARIs reduce LUTS scores by reducing prostate volume by 20% to 30% [5]. The major adverse events (AEs) of 5ARIs include sexual dysfunction, such as erectile dysfunction (ED), decreased libido, and ejaculatory dysfunction (EjD). A large-scale literature review showed that, after 2 years of finasteride therapy, about 12% of patients had to discontinue treatment owing to sexual AEs, thereby confirming the significance of sexual AEs as a major cause of treatment discontinuation [6]. According to existing evidence, sexual AEs occur at similar rates regardless of which 5α-reductase isoform is inhibited [7]. Sexual AEs occur most frequently early in therapy, and seem to decline over time, in patients using either dutasteride or finasteride [8-10].
The aim of this study was to investigate the changes in sexual function during a 1-year follow-up period of dutasteride therapy in Korean patients with BPH.
To prospectively evaluate changes in sexual function during 1 year of treatment with dutasteride, we recruited 73 patients with BPH who had an enlarged prostate (total volume ≥30 g), had LUTS (International Prostate Symptom Score [IPSS] >10), and were undergoing combined treatment with α-blockers (tamsulosin or doxazosin) and dutasteride from January 2007 to July 2009. The patients had relatively good erectile function (EF), defined as an International Index of Erectile Function (IIEF) EF domain score ≥17, and had sexual intercourse at least once per month. To rule out the independent effect of α-blockers on sexual function, all patients were treated with an α-blocker alone for 1 month before the combined treatment with an α-blocker and dutasteride. During the study period, 55 patients completed the treatment; however, 18 patients dropped out in violation of the study (violation of visit day in 3, no sexual intercourse owing to patient's operation or sickness or partner's illness in 3, sexual dysfunction in 2, and failure to follow-up in 10).
IPSS, peak flow rate, post-void residual urine, IIEF scores, prostate volume by transrectal ultrasonography, and serum prostate-specific antigen (PSA) levels were measured before and periodically during treatment. Sexual function during dutasteride therapy was analyzed by total IIEF scores, EF domain scores (Q1, 2, 3, 4, 5, and 15), sexual intercourse satisfaction scores (Q6, 7, and 8), orgasmic function scores (Q9 and 10), sexual desire scores (Q11 and 12), and overall satisfaction scores (Q13 and 14). Patients with post-void residual urine volume ≥100 ml, PSA levels ≥4 ng/ml, or contraindications for α-blockers and dutasteride were excluded from the study.
Changes in sexual functions were prospectively evaluated at 1 month after treatment with α-blocker and at 1, 3, 6, 9, and 12 months after combined treatment with dutasteride and α-blocker. The possibilities of sexual AEs with both α-blockers and dutasteride were explained before the initiation of medication. SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The IIEF scores between baseline and 1 month after treatment with α-blocker, between baseline and throughout combined treatment with dutasteride and α-blocker, and between 1 month after treatment with α-blocker and throughout combined treatment with dutasteride and α-blocker were compared by using the paired t-test. Correlations between changes in IIEF scores and changes in IPSS scores, prostate volume, and PSA levels were analyzed by using the Pearson's correlation test. A p-value less than 0.05 was set as statistically significant.
The average age of the patients was 62.3±7.2 years. The mean pretreatment PSA level and prostate volume were 2.2±1.5 ng/ml and 48.9±16.0 g, respectively (Table 1). IPSS and quality of life were significantly improved and prostate volume was significantly reduced at 12 months after treatment with dutasteride (Table 2).
After 1 month of treatment with alpha-blockers alone, for both tamsulosin and doxazosin, no significant changes in IIEF scores from the pretreatment level were shown. However, the IIEF scores showed the most significant reduction at 1 month after combined treatment with an α-blocker and dutasteride (p<0.01) and then gradually recovered, although EF was still significantly decreased at 12 months (p<0.05) (Table 3).
We studied the changes in each domain score of the IIEF after adding dutasteride to the α-blocker treatment. The EF and overall satisfaction domain scores showed the most significant decrease at 1 month after treatment (p<0.01) and gradually recovered thereafter, but were still significantly decreased at 12 months (p<0.05). Moderate to severe ED (EF score <17) was seen in 38% of patients at 1 month after treatment and in 22% of patients at 12 months after treatment (Fig. 1, 2). The scores for intercourse satisfaction, orgasmic function, and sexual desire also showed the most significant reduction at 1 month after treatment, but they were restored to baseline levels at 6 months after treatment.
No significant correlation was observed between the change in each sexual function and those in PSA level, prostate volume, or IPSS scores.
To enable BPH development, testosterone must be converted to its active form, DHT, by the enzyme 5-alpha-reductase. DHT levels in the prostate remain normal despite the reduction in testosterone that occurs with aging [11]. Two genes have been identified that encode for the isoenzymes 5-alpha-reductase types 1 and 2. The type 1 isoenzyme is found in the liver at birth and in two waves in the skin: from just before birth until 2 or 3 years of age and then again from puberty through adulthood. More recently, type 1 mRNA has also been found in prostate tissue. The type 2 isoenzyme is found in all prostate tissue, seminal vesicles, epididymis, fetal genital skin, and in the skin and scalp from just before birth until 2 or 3 years of age as well as in the adult liver [12].
Inhibiting the conversion of testosterone to DHT in peripheral tissues may have implications for the treatment of BPH. Finasteride inhibits 5-alpha-reductase type 2, with little affinity for type 1. It has been shown to be effective in lowering plasma DHT levels by approximately 75% to 80%, leading to a reduction in prostate volume and improved symptoms in men with BPH [11]. Dutasteride inhibits both type 1 and type 2 5-alpha-reductase isoenzymes, reducing circulating DHT by more than 90% [13].
Although the efficacy and safety of 5ARIs for the treatment of enlarged prostates have been well documented, sexual AEs have been reported. ED was the most common sexual AE of finasteride. In a meta-analysis conducted by the American Urological Association (AUA) BPH guidelines committee, the rate of ED in finasteride-treated groups was 8%, compared with 4% in patients treated with placebo [14]. One study reported that 6 months of finasteride therapy caused ED in 22% of patients at 3 months and 33% at 6 months [15].
EjD and decreased libido are the most common sexual AEs after ED. According to the same meta-analysis by the AUA guideline committee, the rate of decreased libido and EjD were 5% vs. placebo 3% and 4% vs. placebo 1%, respectively [14].
The sexual AE profile of dutasteride appears to be similar to that of finasteride with regards to ED, EjD, and decreased libido [7]. In a direct comparative study of 1 year duration, 1630 patients with LUTS taking dutasteride and finasteride experienced comparable incidences of ED (7% vs. 8%, respectively), decreased libido (5% vs. 6%), and EjD (1% vs. 1%) [16]. In this study, moderate to severe ED was experienced in 38% of patients at 1 month and in 22% of patients at 12 months after treatment initiation.
The pathophysiology of sexual function produced by 5ARIs may be related to reduction of DHT levels. In an animal study, Park et al reported that DHT improved gene expression of nitric oxide synthase (NOS) in the corpus cavernosum and played an important role in maintaining NOS activity [17]. DHT is more potent than testosterone in increasing NOS activity in rats [17]. A report demonstrated that 5ARIs caused reduction in NOS activity in the corpus cavernosum and reduction of the erectile response to electrical stimulation [18]. However, Mondaini et al suggested a noxious effect on the basis of the observation that blind administration of finasteride was associated with a significantly higher proportion of sexual dysfunction in patients counseled about potential sexual AEs than in those from whom the same information was withheld [19].
One might assume that a positive correlation would exist between length of therapy and degree of sexual dysfunction. However, in patients using either dutasteride or finasteride, sexual AEs occur most frequently early in therapy and appear to decline over time. The Proscar Long Term Efficacy and Safety Study (PLESS) reported a prevalence of 22% of one or more sexual AEs during the first year of treatment [8]. The difference between the finasteride group and placebo disappeared, with the exception of decreased ejaculatory volume [9]. In one study, patients who received dutasteride for 48 months reported reduction in the incidence of drug-related sexual dysfunctions to less than 0.5% by the end of the fourth year [8]. In this study, dutasteride therapy resulted in the most significant reduction of every sexual function domain score at 1 month after treatment. Overall sexual function recovery was observed at 3 months after treatment, and orgasmic function and sexual desire were restored to the baseline level at 6 months after treatment. However, EF was still significantly reduced at 12 months after treatment.
Testosterone is associated not only with EF, but also with libido and semen production. Likewise, DHT may be related to libido and appears to regulate both semen volume and viscosity through its action on the development and function of the prostate and seminal vesicles [20,21]. 5ARIs reduce blood DHT levels by over 70% but induce a compensatory rise in testosterone level by 20% [22]. Future research is needed to either confirm or refute the hypothesis that a reduction in the DHT level has a greater impact on sexual function than the compensatory increase in testosterone level and to explain why sexual AEs occur early in 5ARI therapy and decline over the duration of treatment.
Dutasteride therapy resulted in the most significant reduction in all IIEF sexual function domain scores at 1 month after treatment. Overall recovery in sexual function was noted after 3 months of treatment, and orgasmic function and sexual desire were restored to baseline levels after 6 months of treatment. However, the EF score was still significantly reduced at 12 months. No significant correlation was observed between changes in sexual function and IPSS, serum PSA level, or prostate volume.
Figures and Tables
FIG. 1
Changes in International Index of Erectile Functionerectile function score during 12 months of treatment.

FIG. 2
Percentile of patients with moderate to severe erectile dysfunction (ED) during 12 months of treatment according to International Index of Erectile Function-erectile function score.
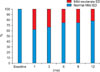
TABLE 2
International Prostate Symptom Score (IPSS), uroflow rate, and prostate volume at baseline and after 12 months of treatment with dutasteride

References
1. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998. 97:1837–1847.
2. Chung BH, Lee JY, Lee SH, Yoo SJ, Lee SW, Oh CY. Safety and efficacy of the simultaneous administration of udenafil and an alpha-blocker in men with erectile dysfunction concomitant with BPH/LUTS. Int J Impot Res. 2009. 21:122–128.
3. Nickel JC, Elhilali M, Emberton M, Vallancien G. Alf-One Study Group. The beneficial effect of alfuzosin 10 mg once daily in 'real-life' practice on lower urinary tract symptoms (LUTS), quality of life and sexual dysfunction in men with LUTS and painful ejaculation. BJU Int. 2006. 97:1242–1246.
4. Iehlé C, Délos S, Guirou O, Tate R, Raynaud JP, Martin PM. Human prostatic steroid 5 alpha-reductase isoforms--a comparative study of selective inhibitors. J Steroid Biochem Mol Biol. 1995. 54:273–279.
5. Marihart S, Harik M, Djavan B. Dutasteride: a review of current data on a novel dual inhibitor of 5alpha reductase. Rev Urol. 2005. 7:203–210.
6. Edwards JE, Moore RA. Finasteride in the treatment of clinical benign prostatic hyperplasia: a systematic review of randomised trials. BMC Urol. 2002. 2:14.
7. Erdemir F, Harbin A, Hellstrom WJ. 5-alpha reductase inhibitors and erectile dysfunction: the connection. J Sex Med. 2008. 5:2917–2924.
8. Wessells H, Roy J, Bannow J, Grayhack J, Matsumoto AM, Tenover L, et al. Incidence and severity of sexual adverse experiences in finasteride and placebo-treated men with benign prostatic hyperplasia. Urology. 2003. 61:579–584.
9. Stoner E. Three-year safety and efficacy data on the use of finasteride in the treatment of benign prostatic hyperplasia. Urology. 1994. 43:284–292.
10. Roehrborn CG, Marks LS, Fenter T, Freedman S, Tuttle J, Gittleman M, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology. 2004. 63:709–715.
11. Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, et al. The effect of finasteride in men with benign prostatic hyperplasia. 1992. J Urol. 2002. 167:1102–1107.
12. Sawaya ME, Price VH. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997. 109:296–300.
13. Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. Eur Urol. 2000. 37:367–380.
14. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003. 170:530–547.
15. Uygur MC, Arik AI, Altuğ U, Erol D. Effects of the 5 alpha-reductase inhibitor finasteride on serum levels of gonadal, adrenal, and hypophyseal hormones and its clinical significance: a prospective clinical study. Steroids. 1998. 63:208–213.
16. Andriole GL, Kirby R. Safety and tolerability of the dual 5alpha-reductase inhibitor dutasteride in the treatment of benign prostatic hyperplasia. Eur Urol. 2003. 44:82–88.
17. Park KH, Kim SW, Kim KD, Paick JS. Effects of androgens on the expression of nitric oxide synthase mRNAs in rat corpus cavernosum. BJU Int. 1999. 83:327–333.
18. Seo SI, Kim SW, Paick JS. The effects of androgen on penile reflex, erectile response to electrical stimulation and penile NOS activity in the rat. Asian J Androl. 1999. 1:169–174.
19. Mondaini N, Gontero P, Giubilei G, Lombardi G, Cai T, Gavazzi A, et al. Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J Sex Med. 2007. 4:1708–1712.
20. Riley AJ. Life-long absence of sexual drive in a woman associated with 5-dihydrotestosterone deficiency. J Sex Marital Ther. 1999. 25:73–78.
21. Cai LQ, Fratianni CM, Gautier T, Imperato-McGinley J. Dihydrotestosterone regulation of semen in male pseudohermaphrodites with 5 alpha-reductase-2 deficiency. J Clin Endocrinol Metab. 1994. 79:409–414.
22. Guess HA, Gormley GJ, Stoner E, Oesterling JE. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996. 155:3–9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download