Abstract
Purpose
It is well known that fungi become predominant microorganisms in the urine of patients with long-term Foley catheters. This study was conducted to evaluate the lengths of time for fungi to cause urinary tract infection (UTI) and to identify predictors of fungal UTI in burn patients with long-term Foley catheters.
Materials and Methods
A total of 93 patients who did not have infection at the time of admission but later had fugal UTI were evaluated. Urinalysis, urine culture, and Foley catheter indwelling were done at admission. All patients were administered prophylactic antibiotics from admission. Urine cultures were run every week, and catheters were changed every 2 weeks for each patient.
Results
Three of the 93 patients (3.2%) displayed fungal UTI at the 1st week of catheter indwelling. However, most patients (78.5%) displayed fungal UTI from 2nd to 5th week after catheter indwelling. The most prevalent fungus identified was Candida tropicalis (60.2%). By univariate logistic regression analysis, only the total body surface area burned (TBSAB) was predictive of fungal UTI in burn patients (p=0.010). By multivariate logistic regression analysis, underlying disease (p=0.032) and TBSAB (p=0.036) were predictors of fungal UTI. Patients with higher TBSAB were more likely to display shorter intervals from Foley catheterization to fungal UTI.
Urethral catheterization is generally more disadvantageous than clean intermittent catheterization (CIC). Given that it has more complications than diaper application in incontinence, it should be avoided in patients to the extent possible. However, for reasons relating to convenience in patient management, ignorance of the risks associated with catheterization, and medical inevitability, it is commonly performed.
Even with preventive administration of antibiotics and the most careful hygiene of the urethra and catheter, urinary tract infection (UTI) occurs quite frequently in patients with long-term urethral catheterization. Nosocomial UTI mostly results from direct introduction of urethral microorganisms at the time of catheterization [1,2]. Microorganisms can ascend to the bladder along the outside of the catheter in the periurethral mucosal sheath, between the catheter and the urethral mucosa [1-3], or intraluminally along the internal lumen of the catheter after the collection bag or catheter-drainage tube junction has been contaminated.
Candida species, the most common cause of fungal UTI, are normal commensals in humans. Candidal UTI usually occurs in patients with urinary catheters, typically after bacteriuria and antibiotic therapy, and sometimes bacterial and candidal infections occur simultaneously. The most frequently occurring organism is Candida albicans, followed by Candida glabrata, Candida tropicalis, and Candida krusei [4]. However, non-albicans Candida species and non-candidal yeasts are increasingly being reported as the etiological cause of fungal UTI [5].
Burn patients are susceptible to nosocomial infections owing to the immunocompromising effects of burn injury, cutaneous and respiratory tract injury, prolonged intensive care unit stays (which may involve endotracheal intubation and catheterization of blood vessels and bladder), and broad-spectrum antibiotic therapy. Despite the obvious risk of diverse nosocomial infections, the vast majority of studies on hospital-acquired infections have focused on burn wound infections. Until recently, there have been few reports and studies of urine cultures of burn patients with urinary catheters.
This study was done to evaluate the lengths of time it takes for fungi to cause fungal UTI and what predictors are involved in such occurrence of fungal UTI of burn patients with long-term Foley catheterization. This study is the first of its type in Korea.
A total of 726 burn patients with long-term urethral catheterization who were admitted to our institution's burn center from January 2007 to December 2008 were analyzed retrospectively. Of the 726 patients, 583 patients showed no growth of fungus and 143 (19.7%) patients showed fungal growths from urine, blood, catheter tip, bronchial washing fluid, or sputum. Among these 143 patients, 93 were found to have fungal UTI by urine culture. The 93 patients who did not have any infection at the time of admission but were diagnosed with fungal UTI later during their hospitalization were selected as subjects for our study. The study pool consisted of 55 males and 38 females with a mean age of 51.9 years (range, 25 to 89 years). Urinalysis and urine culture results confirmed that none of the patients had UTI before catheterization. All patients were administered antibiotic injections from the date of admission or were administered oral forms later. Intravenous antibiotics were given until the patients were able to tolerate oral intake. No patients were receiving anti-fungal agents unless they had fungal UTI.
Patients were classified into 3 groups by the percentage of burn: <30%, 30% to 44%, and ≥45% on the basis of the total body surface area burned (TBSAB). TBSAB was estimated by using the Lund and Browder chart [6].
Silicon-coated latex Foley catheters, 16 to 20 French, were inserted aseptically after lubrication by a team of trained health professionals following various procedures. The catheters were changed every 2 weeks.
Urine samples were collected aseptically within 2 hours after insertion of the catheters for baseline urine cultures and microscopic examinations. Thereafter, weekly urine cultures and urinalysis were done. Urine samples were aspirated from the needleless sampling port with a sterile syringe or cannula adapter after cleansing the port with a disinfectant and were immediately sent to the microbiology laboratory. All urine samples were inoculated on blood agar culture medium plates (BAP). The plates were cultured for 48 hours at 35℃ until a few colonies grew more than 1 mm in diameter. After the colonies were verified as fungi by Gram staining, fungal identification was done. The colonies picked from BAP were inoculated and further cultured in CHROMagar culture medium for 24 hours at 35℃ for identification. Also, the microorganisms were collected by centrifugation at 500x g for 5 minutes and the density was adjusted with distilled water to 2.0 McFarland. The colonies were identified and typed by use of VITEK2 YST fungal identification plates.
Fungal UTI in the patients with catheterization was defined as colony counts greater than 104 CFU/ml in urine culture. The interval of catheterization to fungal UTI referred to the time interval from Foley catheterization until the diagnosis of fungal UTI. These intervals were noted by 6 groups on the basis of the number of weeks: 1st week (≤7 days), 2nd week (8-14 days), 3rd week (15-21 days), 4th week (22-28 days), 5th week (29-35 days), and ≥6th week (≥36 days).
SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used for analysis. The chi-square test (with Fisher's exact test) was done to determine whether the TBSAB was associated with the interval of catheterization to fungal UTI. Univariate and multivariate logistic regression analyses were done to determine predictors of fungal UTI. A probability level of p<0.05 was considered significant.
Among the 93 patients, 29 patients (31.2%) had underlying diseases such as diabetes mellitus, hypertension, malignancy, cerebrovascular accident, and spinal injury. Thirty-six patients had TBSAB <30%, 35 patients had TBSAB in the range of 30% to 44%, and 22 patients had TBSAB ≥45%.
Three (3.2%) of 93 patients had fungal UTI at the 1st week of urethral Foley catheterization. Twenty-two (23.7%) of 93 patients had fungal UTI at the 2nd week, 27 patients (29.0%) at the 3rd week, 17 patients (18.3%) at the 4th week, 7 patients (7.5%) at the 5th week, and 17 patients (18.3%) at greater than the 6th week of urethral catheterization (Fig. 1).
Candida tropicalis, the most prevalent fungus, was isolated in 56 patients (60.2%). Candia albicans was identified in 26 patients (28.0%), Candida glabrata in 8 patients (8.6%), Candia parapsilosis in 2 patients (2.2%), and Trichosporon asahii in 1 patient (1.1%).
Regarding the three groups classified by % TBSAB, none of the 36 patients with TBSAB <30%, only 1 (2.8%) of 35 patients with TBSAB in the range of 30% to 44%, and 2 (9.0%) of 22 patients with TBSAB ≥ 45% developed fungal UTI within the 1st week (Fig. 2). Each set of data at the 2nd, 3rd, 4th, 5th, and ≥6th week are described in the same way as the above-mentioned data for the 1st week. The intervals of catheterization to fungal UTI had different distributions according to the TBSAB, and patients with higher TBSAB were more likely to display shorter intervals from Foley catheterization to fungal UTI.
The results indicated that intervals of catheterization to fungal infection were related to TBSAB (Fisher's exact test, p=0.005) (Fig. 2). The peak incidence of fungal UTI occurred at the 4th week of Foley catheterization in the <30% TBSAB group, at the 2nd to 3rd week in the 30% to 44% TBSAB group, and at the 3rd week in the ≥45% TBSAB group.
Univariate and multivariate logistic regression analyses were performed to determine the predictors of fungal UTI in burn patients with indwelling urethral Foley catheters (Table 1, 2). According to the univariate logistic regression analysis, only TBSAB was predictive of fungal UTI in burn patients (p=0.010). Age, sex, underlying disease, kinds of burn, and kinds of fungi were not associated with fungal UTI. According to the multivariate logistic regression analysis, underlying disease and TBSAB were predictors of fungal UTI in burn patients with indwelling urethral Foley catheters. Patients with underlying disease were associated with a higher risk of fungal UTI (odds ratio [OR] 4.201, p=0.032) than were patients without underlying disease. With respect to TBSAB, patients with higher TBSAB were more likely to have shorter intervals from catheterization to fungal UTI (p=0.036). Patients with TBSAB in the range of 30% to 44% were associated with a shorter interval from catheterization to fungal UTI (OR 0.231), and those with TBSAB ≥45% were associated with the shortest interval (OR 0.150) compared with patients with TBSAB <30%.
In the management of burn patients, infection with systemic sepsis is the most important problem. This systemic sepsis resulting from invasive infection remains the leading cause of death among patients hospitalized with major thermal injury. One of these is urosepsis. Most urosepsis in burn patients is catheter-related UTI. The flora of individual burn wounds changes over time: gram-negative organisms gradually replace the gram-positive [7,8]. In addition, gram-negative bacilli, which were once common colonizers of burn wounds and consequently common causes of nosocomial infections in burn patients, have become less common in recent years owing to the use of topical antibiotics and effective systemic antibiotics, particularly for Pseudomonas species [9]. Many studies of nosocomial infections have shown an increase in fungal isolates, particularly Candida species. Data from the National Nosocomial Infection Surveillance System of the United States indicated that the rate of nosocomial fungal infections was highest in burn and trauma wards [10]. However, nearly all of these studies were related to burn wounds, not UTI or urine cultures. Until recently, there have been few reports or studies of fungal growth in the urine cultures of burn patients with long-term Foley catheters.
We found fungal UTI (3/93, 3.2%) in burn patients at the 1st week of urinary catheterization. In the reported studies [11,12], most of the fungal growth in wounds, blood, sputum, and catheter tips started more than 2 weeks after the thermal injury in burn patients. A possible explanation for this might be that a 2-week period is necessary for exogenous fungal colonization on wounds or other sites or from the patient's own gastrointestinal or upper respiratory tract flora [13]. In our study, antibiotics with a strong effect and extensive spectrum were always administered empirically during the early phase after the burn injury (at the time of admission) and this might be why the fungal UTI began to be found in burn patients within the 1st week. Even though 3 cases were found within the 1st week, all of them happened at the 6th day of urinary catheter indwelling. These facts suggest that fungal UTI seems to begin at around the 1st week of urinary catheter indwelling.
It was previously reported that the main pathogenic fungal types are candidal, aspergilli, and cryptococcal [14-16]. Although Candida species were reported as the most common fungi of invasive infection some decades ago, more non-candidal fungal strains and conditional pathogenic fungi, such as some strains of yeast, saprogen, and others, have been isolated in invasive fungal wound infections in recent years [17-19]. In this study, we found that Candida tropicalis (60.2%) and Candida albicans (28.0%) were the two main types of fungi in the fungal UTI of burn patients, whereas Candida albicans (74%), Candida glabrata (8%), Candida parapsilosis (7%), and Candida tropicalis (3%) were cultured in urine of non-thermal injury patients with catheter indwelling in the study of Richardson and Warnock [20].
Previous reports documented predisposing factors as well as other associated predisposing causes, including papillary necrosis, prematurity, and congenital anomalies [21-27]. Established predisposing factors for candidal urinary tract infections in patients without burns are diabetes mellitus, female gender, prolonged catheter drainage, and corticosteroid use [23,28]. However, these studies were conducted in patients with no burns. The predisposing factors in our study were underlying disease and TBSAB. The higher the TBSAB, the shorter the time interval of catheterization to fungal UTI.
Bloodstream infection, pneumonia, burn wound infection, and urinary tract infection, among others, have been cited as specific sites of infection that are particularly important for burn patients. Fever, a highly specific indicator of infection for many patient populations, has frequently been found to not be well correlated with the presence of infection in burn patients because of core temperature increases and an increase in heat production associated with the onset of a hypermetabolic response [29]. Consequently, fever alone, absent other signs and symptoms, is often not indicative of infection in burn patients. In addition, it is difficult to gauge burn wound sepsis by clinical signs and symptoms, and the best way to make a diagnosis is through careful serial evaluation of the patient. Furthermore, culturing and surveillance guidelines need to be more stringent for burn patients, especially in those having large TBSAB given the higher likelihood of infection and its transmission [30]. With respect to these points, our observations help many clinicians predict fungal UTI in burn patients with urethral Foley catheters.
Fungal UTI in burn patients with Foley catheters was initially found at the 1st week of urinary catheterization, showing the highest peak incidences at the 2nd to 3rd week. The most prevalent fungus was Candida tropicalis. Time intervals from catheterization to fungal UTI in these patients were shortened by the presence of underlying disease and higher TBSAB.
Figures and Tables
FIG. 1
Time intervals from catheterization to fungal urinary tract infection (UTI) in burn patients. Three (3.2%) of 93 patients had fungal UTI during the 1st week of urethral Foley catheterization. Twenty-two (23.7%) of 93 patients had fungal UTI in the 2nd week, 27 patients (29.0%) in the 3rd week, 17 patients (18.3%) in the 4th week, 7 patients (7.5%) in the 5th week, and 17 patients (18.3%) at greater than the 6th week of urethral catheterization. Fungal UTI began to appear during the first week of urinary catheter indwelling.
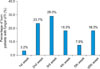
FIG. 2
Time intervals from catheterization to fungal urinary tract infection (UTI) in burn patients according to TBSAB. Each axis stands for the interval from catheterization to fungal UTI on the basis of the number of patients. Three different lines of three shapes of hexagons stand for 3 groups by the percentage of burn: <30%, 30% to 44%, and ≥45% on the basis of total body surface area burned (TBSAB). For example, the axis of the 1st week means that none of the 36 patients with TBSAB <30%, only 1 (2.8%) of 35 patients with TBSAB in the range of 30% to 44%, and 2 (9.0%) of 22 patients with TBSAB ≥45% developed fungal UTI within the 1st week. As indicated in the figure, the peak incidence of fungal UTI occurred during the 4th week of Foley catheterization in the <30% TBSAB group, at the 2nd to 3rd week in the 30% to 44% TBSAB group, and at the 3rd week in the ≥45% TBSAB group. The intervals of catheterization to fungal UTI were related to TBSAB (Fisher's exact test, p=0.005).

References
1. Kunin CM, McCormack RC. Prevention of catheter-induced urinary tract infections by sterile closed drainage. N Engl J Med. 1966. 274:1155–1161.
2. Maskell R, Pead L, Allen J. The puzzle of "urethral syndrome": a possible answer? Lancet. 1979. 1:1058–1059.
3. Chan RC, Bruce AW, Reid G. Adherence of cervical, vaginal and distal urethral normal microbial flora to human uroepithelial cells and the inhibition of adherence of gram-negative uropathogens by competitive exclusion. J Urol. 1984. 131:596–601.
4. Lundstrom T, Sobel J. Nosocomial candiduria: a review. Clin Infect Dis. 2001. 32:1602–1607.
5. Sobel JD. Management of asymptomatic candiduria. Int J Antimicrob Agents. 1999. 11:285–288.
6. Neaman KC, Andres LA, McClure AM, Burton ME, Kemmeter PR, Ford RD. A new method for estimation of involved BSAs for obese and normal-weight patients with burn injury. J Burn Care Res. 2011. 32:421–428.
7. Pruitt BA Jr, McManus AT. Opportunistic infections in severely burned patients. Am J Med. 1984. 76:146–154.
8. Manson WL, Pernot PC, Fidler V, Sauer EW, Klasen HJ. Colonization of burns and the duration of hospital stay of severely burned patients. J Hosp Infect. 1992. 22:55–63.
9. Pruitt BA Jr, Lindberg RB, McManus WF, Mason AD Jr. Current approach to prevention and treatment of Pseudomonas aeruginosa infections in burned patients. Rev Infect Dis. 1983. 5:Suppl 5. S889–S897.
10. Beck-Sagué CM, Jarvis WR. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. The National Nosocomial Infections Surveillance System. J Infect Dis. 1993. 167:1247–1251.
11. Burdge JJ, Rea F, Ayers L. Noncandidal, fungal infections of the burn wound. J Burn Care Rehabil. 1988. 9:599–601.
12. Schofield CM, Murray CK, Horvath EE, Cancio LC, Kim SH, Wolf SE, et al. Correlation of culture with histopathology in fungal burn wound colonization and infection. Burns. 2007. 33:341–346.
13. Luo G, Peng Y, Yuan Z, Cheng W, Wu J, Fitzgerald M. Yeast from burn patients at a major burn centre of China. Burns. 2011. 37:299–303.
14. Atiyeh BS, Gunns W, Hayek SN. State of the art in burn treatment. World J Surg. 2005. 29:131–148.
15. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006. 19:403–434.
16. Singh V, Devgan L, Bhat S, Milner SM. The pathogenesis of burn wound conversion. Ann Plast Surg. 2007. 59:109–115.
17. Bruck HM, Nash G, Stein JM, Lindberg RB. Studies on the occurrence and significance of yeasts and fungi in the burn wound. Ann Surg. 1972. 176:108–110.
18. Cawley MJ, Braxton GR, Haith LR, Reilly KJ, Guilday RE, Patton ML. Trichosporon beigelii infection: experience in a regional burn center. Burns. 2000. 26:483–486.
19. Holzheimer RG, Dralle H. Management of mycoses in surgical patients - review of the literature. Eur J Med Res. 2002. 7:200–226.
20. Richardson MD, Warnock DW. Richardson MD, Warnock DW, editors. Invasive candidosis. Fungal infection: diagnosis and management. 2003. 3rd ed. Oxford: Blackwell Scientific Publications;192–193.
21. Kim DK, Yoo ES, Kim GN, Chung SK. Emphysematous cystitis with fungus Ball. Korean J Urol. 2002. 43:904–906.
22. Lee SW. An aspergilloma mistaken for a pelviureteral stone on nonenhanced CT: a fungal bezoar causing ureteral obstruction. Korean J Urol. 2010. 51:216–218.
23. Wise GJ, Silver DA. Fungal infections of the genitourinary system. J Urol. 1993. 149:1377–1388.
24. Frye KR, Donovan JM, Drach GW. Torulopsis glabrata urinary infections: a review. J Urol. 1988. 139:1245–1249.
25. Bell DA, Rose SC, Starr NK, Jaffe RB, Miller FJ Jr. Percutaneous nephrostomy for nonoperative management of fungal urinary tract infections. J Vasc Interv Radiol. 1993. 4:311–315.
26. Sonda LP, Amendola MA. Candida pyocalix: unusual complication of prolonged nephrostomy drainage. J Urol. 1985. 134:722–724.
27. Vordermark JS 2nd, Modarelli RO, Buck AS. Torulopsis pyelonephritis associated with papillary necrosis: a case report. J Urol. 1980. 123:96–97.
28. Wainstein MA, Graham RC Jr, Resnick MI. Predisposing factors of systemic fungal infections of the genitourinary tract. J Urol. 1995. 154:160–163.
29. Çakir B, Yeğan BÇ. Systemic responses to burn injury. Turk J Med Sci. 2004. 34:215–226.
30. Tredget EE, Shankowsky HA, Rennie R, Burrell RE, Logsetty S. Pseudomonas infections in the thermally injured patient. Burns. 2004. 30:3–26.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download