Abstract
Sexually transmitted diseases (STDs) are the most common infectious diseases worldwide, with over 350 million new cases occurring each year, and have far-reaching health, social, and economic consequences. Failure to diagnose and treat STDs at an early stage may result in serious complications and sequelae. STDs are passed from person to person primarily by sexual contact and are classified into varied groups. Some cause mild, acute symptoms and some are life-threatening. They are caused by many different infectious organisms and are treated in different ways. Syphilis and gonorrhea are ancient afflictions. Now, however, Chlamydia is prevalent and has become the most common bacterial STD. Antimicrobial resistance of several sexually transmitted pathogens is increasing, rendering some regimens ineffective, adding to therapeutic problems. A standardized treatment protocol for STDs is recommended to ensure that all patients receive adequate treatment. Appropriate treatment of STDs is an important public health measure.
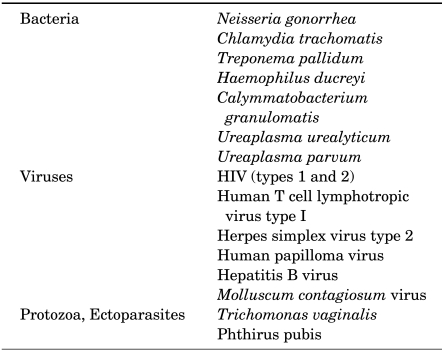
Sexually transmitted diseases (STDs) are clinical syndromes caused by pathogens that can be acquired and transmitted through sexual activity. The pathogens of STDs are bacteria, viruses, fungi, protozoa, and epizoa (Table 1). Some pathogens can be sexually transmissible even when nonsexual routes of transmission predominate. The total number of sexually transmissible pathogens now exceeds 35, and each pathogen may have multiple subtypes that may have differing clinical manifestations.
The overall incidence and prevalence of bacterial STDs, in particular gonorrhea, syphilis, chancroid, and chlamydial infections, have declined since World War II. However, the incidence of STDs is still high in developing countries, and developing countries are disproportionately affected [1,2]. STDs are not spread randomly; unprotected sex with an infected partner is by far the most important risk factor [1]. The prevalence and distribution of infection depends on the behavior of an individual and his or her sex partners. Globally, an estimated 12 million people are infected every year, and the majority of infections are believed to occur in developing countries [3].
If the diagnosis and treatment of STDs are delayed or inadequate, the burden of morbidity is significant, especially among women. The most serious complication in women is pelvic inflammatory disease, primarily associated with chlamydia and gonorrhea [4]. Chlamydia, gonorrhea, syphilis, and chancroid can be thought of as curable STDs, which as a group appear to be short-lived with a high transmission probability [5-7], and will be discussed in this review article.
The major pathogen causing nongonococcal urethritis (NGU) is Chlamydia trachomatis (C. trachomatis), which is estimated to account for 30% to 50% of cases. Chlamydial genital infection is the most frequently reported infectious disease in STD clinics, and the prevalence is highest in persons aged under 35 years [8-10]. Asymptomatic infection is common in women, but approximately 70% of men have symptoms such as urethral discharge, dysuria, penile irritation, and signs of epididymo-orchitis or prostatitis.
C. trachomatis is an obligate intracellular parasite and cannot be cultured on artificial media [11]. Chlamydial infections of the genital tract are primarily caused by serovars D, E, F, G, H, I, J, and K [11]. Among rectal infections in homosexual men, the D and G serovars are particularly prevalent [12].
Chlamydial infections of the genital tract have a worldwide distribution. The prevalence of chlamydial urethral infection ranges from 3% to 5% of asymptomatic men in general medical clinics to 15% to 20% of all men in STD clinics [13-15].
Chlamydial infection is a cause of acute proctitis in homosexual men who practice receptive rectal intercourse, and pharyngeal infection with C. trachomatis has been demonstrated in 3% to 6% of men and women with a history of recent orogenital contact [16,17]. Chlamydial genital infections are closely related to infection with gonorrhea in clinical manifestations. Both organisms can infect the transitional epithelium of the urethra and can extend to the epididymis, the endocervix, the endometrium, salpinx, peritoneum, and the rectum [18,19]. They can produce extensive subepithelial inflammation, epithelial ulceration, and scarring.
C. trachomatis causes 35% to 50% of NGU in heterosexual men. Clinically, chlamydia-positive and chlamydia-negative NGU cannot be differentiated on the basis of signs or symptoms [20,21]. Both usually present after an incubation period of 7 to 21 days with dysuria and mild-to-moderate whitish or clear urethral discharge. Examination reveals no abnormalities other than the discharge in most cases. Most men with asymptomatic chlamydial urethral infection exhibit persistent urethral leukocytosis on Gram stains of urethral secretions or persistent pyuria in a first-void urine [22].
The patients with NGU probably acquire gonorrhea and chlamydial infection simultaneously, but, because of the longer incubation period of C. trachomatis, postgonococcal chlamydial urethritis may develop after the gonorrhea is treated with an agent that cannot eradicate chlamydia [23]. Among men infected with both chlamydia and Neisseria gonorrhea (N. gonorrhea) who are treated with penicillin, ampicillin, gentamicin, or spectinomycin, 80% or more develop symptomatic postgonococcal chlamydial urethritis or urethral leukocytosis without symptoms [24].
C. trachomatis causes most cases of idiopathic epididymitis in young, heterosexually active males [25]. Chlamydial and gonococcal epididymitis are usually associated with urethritis caused by C. trachomatis or N. gonorrhea in patients who are less than 35 years of age and sexually active. Currently, about 70% of acute epididymitis in young, sexually active men appears to be attributable to chlamydial infection. Chlamydial epididymitis presents as unilateral scrotal pain, swelling, tenderness, and fever. Men with chlamydial epididymitis improve rapidly with tetracycline treatment, supporting the causal role of C. trachomatis [25].
Reiter's syndrome (urethritis, conjunctivitis, arthritis, and characteristic mucocutaneous lesions) had been related to genital infection with C. trachomatis [26].
Many women with chlamydia isolated from the cervix have no signs or symptoms of infection; the most commonly found symptoms are mucopurulent discharge and hypertrophic ectopy [27]. No genital symptoms are specifically correlated with chlamydial cervical infection. Findings on examination suggestive of chlamydial infection include easily induced endocervical bleeding and mucopurulent endocervical discharge. The observation of purulent yellow or greenish cervical discharge on a cervical swab correlates with the presence of chlamydial or gonococcal infection [28].
Because C. trachomatis is an intracellular pathogen, the cell culture was the gold standard test for detection for years [11]. Owing to the inadequacies, cost, and technical difficulties of cell culture, however, many nonculture diagnostic tests have been developed for C. trachomatis [11,29]. The most widely used of these assays are the direct fluorescent antibody (DFA) and enzyme immunoassay (EIA) tests. In general, these tests detect between 60% and 85% of infections relative to culture [29,30].
The advantages of the nonculture tests in comparison with culture are the ease of use in various settings and low cost. However, a noninvasive means of diagnosing chlamydial infection by testing urine has not been realized. The sensitivities of these assays with urine have been unacceptably low, in the range of 40% to 50%, as compared with culture [31]. The specificity of the automated methods for the detection of amplified C. trachomatis DNA or RNA is consistently above 99% [30]. The polymerase chain reaction (PCR) in the diagnosis of chlamydial infection has also been a gold standard. Among males, its sensitivity is generally between 87% and 100% [31,32].
The most active drugs against C. trachomatis in tissue culture are rifampin and the tetracyclines, followed by macrolides, sulfonamides, fluoroquinolones, and clindamycin. Penicillin, ampicillin, cephalosporins, and spectinomycin for treatment of gonorrhea do not eradicate concomitant chlamydial infection, and 7 or more days of treatment with tetracyclines or macrolides can eradicate C. trachomatis. Fluoroquinolones, such as ofloxacin and levofloxacin show similar cure rates, but ciprofloxacin has a relatively high failure rate, so it should not be used for the treatment of NGU [33,34]. Azithromycin has a half-life of 5 to 7 days and excellent intracellular and tissue penetration. It is the only single-dose agent for the treatment of chlamydial infection (Table 2) [35].
The recommended length of therapy for NGU using tetracycline or doxycycline ranges from 7 to 21 days. There is as yet no evidence that prolongation of tetracycline or doxycycline therapy beyond 1 week is necessary [36,37]. Sex partners are treated concurrently.
Chlamydial infection recurs 3 to 6 weeks after treatment in 5% to 10% of cases. Most of the recurrent cases are the same immunotype as the primary pathogenic strain, and almost all cause recurrent clinical evidence of urethritis.
Gonorrhea is one of the oldest known diseases of humans [38]. The major clinical manifestations of gonorrhea in men were described in ancient Chinese, Egyptian, Roman, and Greek literature as well as in the Old Testament [39]. In the fourth and fifth centuries B.C., Hippocrates wrote about gonorrhea and he understood that it resulted from "the pleasures of Venus." In the second century, Galen coined the word gonorrhea, by which he meant "flow of semen." Neisser demonstrated N. gonorrhea in 1879, and Leistikow and Loffler cultured the organism in vitro in 1882 [40,41].
Gonorrhea is the second most commonly reported bacterial STD, and the incidence of new cases of gonorrhea is especially high in developing countries [42-44]. Most infected people, up to 80% of women and 10% of men, are asymptomatic. Co-infections with chlamydia and other STDs are very common. Among women, gonococcal infections might not produce recognizable symptoms until complications such as pelvic inflammatory disease have occurred.
The incidence of gonorrhea varies with age. Seventy-five percent of cases occur in persons aged under 30 years. The incidence of gonorrhea is almost twice as high for sexually active adolescents as for sexually active women in the 20-24-year-old age group [45,46]. Other risk factors are low socioeconomic status, early onset of sexual activity, unmarried marital status, and a history of past gonorrhea [47]. The incidence of gonorrhea in men who have sex with men may still be higher than in exclusively heterosexual persons [48].
N. gonorrhea is the etiologic pathogen of gonorrhea and its related clinical syndromes such as urethritis, cervicitis, salpingitis, bacteremia, arthritis, and others. N. gonorrhea bacteria are gram-negative diplococci, nonmotile and non-spore-forming, that characteristically grow in pairs with adjacent flattened sides [49].
Humans are the only natural host for gonococci. Gonococci survive only a short time outside the human body. There is no evidence that natural transmission occurs from toilet seats or similar objects. Because gonorrhea is an infection spread by contact, immediate physical contact with the mucosal surfaces of an infected sexual partner is required for transmission.
N. gonorrhea initially infects noncornified epithelium, most often of the urogenital tract and secondarily of the rectum, oropharynx, and conjunctivae [50]. Ascending genital infections such as salpingitis, epididymitis, and bacteremia are relatively common and account for most of the serious morbidity due to gonorrhea.
The initial event in gonococcal infection is the adherence of N. gonorrhea to mucosal cells in a process mediated by pili, Opa, and perhaps other surface proteins [51,52]. Progressive mucosal cell damage and submucosal invasion are accompanied by a vigorous polymorphonuclear leukocytic response, submucosal microabscess formation, and exudation of purulent material into the lumen of the infected organ [53].
Clinical gonorrhea is manifested by a broad spectrum of clinical presentations including asymptomatic and symptomatic local infections, local complicated infections, and systemic dissemination.
The risk of acquiring urethral infection for a man following a single episode of vaginal intercourse with an infected woman is estimated to be 20%, rising to an estimated 60% to 80% after four exposures [53]. The prevalence of infection in women named as secondary sexual contacts of men with gonococcal urethritis has been reported to be 50% to 90% [53,54].
The single exposure transmission rate from male to female is higher than that from female to male because of retention of the infected ejaculate within the vagina. Gonorrhea transmission through insertive or receptive rectal intercourse presumably is relatively efficient, and pharyngeal gonorrhea is readily acquired by fellatio [55]. The mean time to development of symptoms is 3.4 days. The incidence of asymptomatic urethral gonococcal infection in the general population has been estimated at approximately 1% to 3% [56].
In men, gonococcal urethritis usually causes more florid signs and symptoms than nongonococcal urethritis and has a more abrupt onset, more prominent dysuria, and a urethral discharge that is more profuse and more purulent in appearance [57]. The incubation period for gonorrhea usually is shorter than that of nongonococcal urethritis.
The most common manifestation of gonococcal infection in men is an acute anterior urethritis. The incubation period ranges from 1 to 14 days or even longer. Symptoms develop in most cases within 2 to 5 days [58].
The predominant symptoms are urethral discharge and dysuria. Initially, urethral discharge is scant and mucoid or mucopurulent, but the urethral exudate becomes frankly purulent and relatively profuse within 24 hours of onset [53]. Approximately 25% of patients develop only a scant or minimally purulent exudate, grossly indistinguishable from the nongonococcal urethritis [56,59], and a minority never develop overt signs [5,56]. Dysuria usually begins after the onset of discharge. Edema and erythema of the urethral meatus are common.
Without treatment, the usual course of gonococcal urethritis is spontaneous resolution over a period of several weeks, and 95% of untreated patients become asymptomatic within 6 months. Subsequent asymptomatic carriage of N. gonorrhea may occur. Complications of gonococcal urethritis include epididymitis, acute or chronic prostatitis, seminal vesiculitis, and infections of Cowper's and Tyson's glands.
The endocervical canal is the primary site of gonococcal infection in women. Urethral colonization is present in 70% to 90% of infected women [60]. Infection of the periurethral gland or Bartholin's gland ducts is also common. The incubation period for urogenital gonorrhea in women is less certain and probably more variable than in men, but most who develop local symptoms apparently do so within 10 days of infection [61]. The most common symptoms are increased vaginal discharge, dysuria, intermenstrual uterine bleeding, and menorrhagia, each of which may occur alone or in combination and may range in intensity from minimal to severe [62]. Purulent exudate occasionally may be expressed from the urethra, periurethral glands, or the Bartholin's gland duct.
The rectal mucosa is a frequent site of infection in homosexual men and is infected in 35% to 50% of women with gonococcal cervicitis. It is the only site of infection in approximately 5% of women with gonorrhea and 40% of homosexually active men [63]. Most rectal infections in women occur without acknowledged rectal sexual contact and result from perineal contamination with infected cervical secretions. In women, rectal gonorrhea is usually asymptomatic. The symptoms of rectal gonococcal infection range from minimal anal pruritus, painless mucopurulent discharge, or scant rectal bleeding to symptoms of overt proctitis, including severe rectal pain, tenesmus, and constipation.
Gonococcal infection is transmitted to the pharynx by orogenital sexual contact and is more efficiently acquired by fellatio than by cunnilingus. Pharyngeal infection occurs in 3% to 7% of heterosexual men, 10% to 20% of heterosexual women, and 10% to 25% of homosexually active men among patients with gonorrhea [64]. Pharyngeal gonococcal infection may cause acute pharyngitis or tonsillitis and occasionally is associated with fever or cervical lymphadenopathy, but over 90% of pharyngeal infections are asymptomatic. The occasional occurrence of symptomatic pharyngitis and a possible increased risk of disseminated gonococcal infection in persons with pharyngeal gonorrhea are countered by the usual absence of symptoms and a spontaneous cure rate that approaches 100% within 12 weeks of infection [64,65].
Specific diagnosis of infection with N. gonorrhea can be performed by testing endocervical, vaginal, urethral, or urine specimens. Culture, nucleic acid hybridization tests, and nucleic acid amplification tests (NAATs) are available for the detection of genitourinary infection with N. gonorrhea [66].
The standard diagnostic procedure for men with symptomatic urethritis is the Gram stain, because of its high specificity (>99%) and sensitivity (>95%) [67]. However, in asymptomatic men or in women with genital infection, the Gram stain is less useful, because of lower sensitivity. Gram stain of endocervical specimens, pharyngeal specimens, or rectal specimens is not sufficient to detect infection and therefore is not recommended.
NAATs allow testing of the widest variety of specimen types including endocervical swabs, vaginal swabs, urethral swabs in men, and urine from both men and women. The sensitivity of NAATs for the detection of N. gonorrhea in genital and nongenital anatomical sites is superior to culture but varies by NAAT type [68]. All persons found to have gonorrhea should also be tested for other STDs, including chlamydia, syphilis, and HIV [69].
By the late 1980s, penicillins and tetracyclines were no longer recommended for gonorrhea therapy. Because of the high prevalence of penicillinase producing N. gonorrhea (PPNG), in 1981, spectinomycin had been adopted as the drug of choice for gonorrhea therapy. In 1987, clinically significant chromosomally mediated resistance to spectinomycin was reported and the prevalence of spectinomycin-resistant N. gonorrhea increased [70]. In 1993, fluoroquinolones were recommended for the therapy of gonorrhea. These were effective in single-dose, oral regimens and were unrelated to β-lactam antibiotics. By 2007, however, the prevalence of gonococcal strains with diminished fluoroquinolone susceptibility had exceeded 5% [71].
Now ceftriaxone and other third-generation cephalosporin antibiotics have become the most reliable single-dose regimen for the treatment of gonorrhea. The dose of ceftriaxone currently recommended for therapy of uncomplicated gonorrhea is a single intramuscular injection of 250 mg. It is also highly effective for rectal and pharyngeal gonorrhea. Cefixime, an orally absorbed cephalosporin for N. gonorrhea that is similar to ceftriaxone, provides a useful oral alternative to parenteral ceftriaxone. A single 2 g dose of azithromycin has also been found to be effective for gonorrhea therapy [72], although gastrointestinal side effects (nausea, vomiting) are relatively common with this regimen and there is reason for concern for the potential for N. gonorrhea to rapidly develop clinically significant resistance (Table 3).
Patients diagnosed with uncomplicated gonorrhea who are treated with any of the recommended or alternative regimens do not need a test-of-cure, because all recommended regimens have cure rates that approach 100%. Patients who have symptoms that persist after treatment should be evaluated by culture for N. gonorrhea, and any gonococci isolated should be tested for antimicrobial susceptibility.
Syphilis is a systemic disease caused by Treponema pallidum and is the most fascinating disease of humans. On the basis of clinical findings, the disease has been divided into a series of stages, such as primary infection (ulcer or chancre), secondary infection (skin rash, mucocutaneous lesions, and lymphadenopathy), neurologic infection (cranial nerve dysfunction, meningitis, stroke, altered mental status, loss of vibration sense, and auditory or ophthalmic abnormalities), tertiary infection (cardiac or gummatous lesions), and latent infections of unknown duration.
The Treponema species are members of the family Spirochaetaceae within the order Spirochaetales. All members of the Spirochaetales are characterized by spiral shape, corkscrew motility, and the existence of periplasmic flagella [73].
The disease is usually acquired by sexual contact, with the exception of congenital syphilis. Transmission by sexual contact requires exposure to moist mucosal or cutaneous lesions of primary or secondary syphilis. Patients with untreated disease may apparently recover only to relapse over a period of up to 2 years. The rate of acquisition of syphilis from an infected sexual partner during a single sexual contact has been estimated at about 30% [74].
The incidence of syphilis is higher in many other areas of the world than in Western Europe and the United States. About 4 million new cases occur yearly in each of Southeast Asia and sub-Saharan Africa [75].
Darkfield examinations and tests to detect T. pallidum in lesion exudate or tissue are the definitive methods for diagnosing early syphilis [76]. A presumptive diagnosis of syphilis is possible with the use of two types of serologic tests: 1) nontreponemal tests, including Venereal Disease Research Laboratory (VDRL) and Rapid Plasma Reagin (RPR), and 2) treponemal tests, including fluorescent treponemal antibody absorption tests, the Treponema pallidum Hemagglutination Assay, various EIAs, and chemiluminescence immunoassays. The use of only one type of serologic test is insufficient for diagnosis, because each type of test has limitations, including the possibility of false-positive results. False-positive nontreponemal test results can be associated with various medical conditions unrelated to syphilis, including autoimmune conditions, older age, and injection-drug use [76,77]. Therefore, persons with a reactive nontreponemal test should receive a treponemal test to confirm the diagnosis of syphilis.
Nontreponemal test antibody titers may correlate with disease activity, and the results should be reported quantitatively [76]. A fourfold change in titer, equivalent to a change of two dilutions (e.g., from 1 : 16 to 1 : 4 or from 1 : 8 to 1 : 32), is considered necessary to demonstrate a clinically significant difference between two nontreponemal test results that were obtained by using the same serologic test. Sequential serologic tests in individual patients should be performed by using the same testing method, preferably by the same laboratory. The VDRL and RPR are equally valid assays. Nontreponemal test titers usually decline after treatment and might become nonreactive with time.
Most patients who have reactive treponemal tests will have reactive tests for the remainder of their lives, regardless of treatment or disease activity [78]. Treponemal test antibody titers should not be used to assess treatment response.
The initial lesion of syphilis is a papule that appears at the site of venereal contact, from 10 to 90 days, an average of 3 weeks after exposure. The papule grows to a size of 0.5 to 1.5 cm in diameter and after about a week ulcerates, producing the typical chancre of primary syphilis, a round or slightly elongated ulcer, 1 to 2 cm across, with an indurated margin. The ulcer has a clear base, without an exudate. Primary syphilitic chancres most frequently occur in the genital, perineal, or anal area, but any part of the body can be affected. Most chancres are found on the penis of men and on the labia, fourchette, and cervix of women. In the absence of treatment, syphilitic chancres heal spontaneously within 3 to 6 weeks. Modest enlargement of inguinal lymph nodes, frequently bilaterally, is observed.
Differentiating a syphilitic chancre from chancroid, the lesion caused by Haemophilus ducreyi, may be impossible on clinical grounds, although a great degree of tenderness, a jagged border, a yellow exudate, or striking inguinal lymphadenopathy, especially if the overlying skin is thin and shiny, is suggestive of chancroid [79].
Secondary syphilis is a systemic disease. Within a few weeks or months, a variable systemic illness develops, characterized by low-grade fever, malaise, sore throat, headache, adenopathy, and cutaneous or mucosal rash [80].
The initial finding in disseminated syphilis is an evanescent copper-colored macular rash. A few days later, a symmetric papular eruption appears, involving the entire trunk and the extremities, including the palms of the hands and soles of the feet. The papules are red or reddish brown, discrete, and usually 0.5 to 2 cm in diameter. They are generally scaly, although they may be smooth, follicular, or, rarely, pustular. Hypo- or hyperpigmentation may be seen. Alopecia occurs in some cases. Mucosal lesions, either small, superficial, ulcerated areas with grayish borders that resemble painless aphthous ulcers or larger gray plaques, are also common. Large, raised, whitish or gray condyloma lata results from the effects of local skin breakdown in warm, moist areas, most frequently, the axilla and groin.
There may be an interval of 1 to over 20 years from the acute infection to clinical onset of the late or tertiary stages of disease, long after the lesions of early syphilis have been forgotten. By definition, persons with historical or serological evidence of syphilis who have never received treatment for this disease and who have no clinical manifestations are said to have latent syphilis.
Tertiary syphilis occurs in many clinical syndromes, most conveniently divided into three groups: neurosyphilis, cardiovascular syphilis, and late benign syphilis. Abnormalities in the cerebrospinal fluid have been noted in 13% of patients with untreated primary syphilis and in 25% to 40% of patients with untreated secondary syphilis [81].
Syphilis of the cardiovascular system becomes clinically manifest after a latent period of 15 to 30 years. Most patients are between 40 and 55 years of age at onset, and men are affected three times as often as women. Late benign syphilis or gumma is a proliferative granulomatous inflammatory process that may destroy affected tissues. Most lesions occur in the skin and the bones [82].
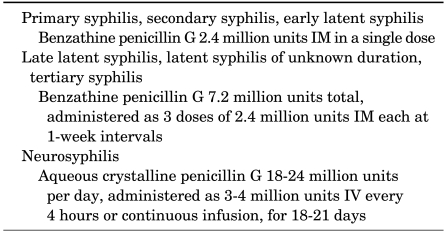
Penicillin G, administered parenterally, is the preferred drug for treating all stages of syphilis. The preparation used, the dosage, and the length of treatment depend on the stage and clinical manifestations of the disease (Table 4) [83,84].
The Jarisch-Herxheimer reaction is an acute febrile reaction frequently accompanied by headache, myalgia, fever, and other symptoms that usually occur within the first 24 hours after the initiation of any therapy for syphilis [85]. Antipyretics can be used to manage symptoms, but they have not been proven to prevent this reaction.
A single dose of 2.4 million units of benzathine penicillin appears to produce a clinical cure of primary or secondary syphilis. For patients who are unable to take penicillin, ceftriaxone 1 g intravenously or intramuscularly every day for 10 days should provide identical results [86]. Tetracycline 500 mg four times daily or doxycycline 100 mg twice daily for 14 days may be just as effective [87]. Azithromycin has been used successfully to treat syphilis and has the advantage of apparently being curative in primary and secondary stage illness after a single oral dose of 1 or 2 g [88]. Patients who fail therapy, as measured by clinical relapse or a fourfold or greater rise in RPR titers, should be retreated, usually with benzathine penicillin in a dose of 2.4 million units IM weekly for 3 weeks [89].
Because latent syphilis is not transmitted sexually, the objective of treating patients with this stage of disease is to prevent complications. A single injection of benzathine penicillin, 2.4 million units, should be given in early latent syphilis (less than 1 year), but three injections should be given at weekly intervals for late latent syphilis or syphilis of unknown duration [91].
The response of latent syphilis to therapy is difficult to assess. Because the patient is free of symptoms, the only possible response is a change in serologic test results. The RPR titer often remains unchanged, or it may decline, but it usually does not return to negative. Rising titers suggest therapeutic failure, in which case a cerebrospinal fluid examination must be done and the patient must receive either a repeat of the initial therapy or a more aggressive therapy.
Haemophilus ducreyi is a gram-negative coccobacillus that causes chancroid, which is characterized by painful genital ulcers and inguinal lymphadenitis [92]. Erythematous papules form at each entry site within several hours to days and evolve into pustules in 2 to 3 days. Papules and pustules are usually not painful. After a few days to 2 weeks, the pustules ulcerate and patients typically have 1 to 4 painful ulcers [92,93].
The combination of a painful genital ulcer and tender suppurative inguinal adenopathy suggests the diagnosis of chancroid [94]. Culture and PCR-based tests are the cornerstones of diagnostic testing.
Chancroid is successfully treated with macrolides, quinolones, and third-generation cephalosporins (Table 5) [93]. Effective regimens are a single dose of azithromycin 1 g orally or ceftriaxone 250 mg intramuscularly, ciprofloxacin 500 mg orally twice a day for 3 days, or erythromycin base 500 mg orally three times a day for 7 days. In general, most of these regimens have cure rates greater than 90% [95,96].
References
1. Aral S, Over M, Manhart L, Holmes KK. Jamison D, Evans D, Alleyne G, Jha P, Breman J, Measham A, editors. Sexually transmitted infections. Disease control priorities in developing countries. 2006. Washington, D.C: World Bank and Oxford University Press;p. 311–330.
2. Wellings K, Collumbien M, Slaymaker E, Singh S, Hodges Z, Patel D, et al. Sexual behaviour in context: a global perspective. Lancet. 2006; 368:1706–1728. PMID: 17098090.

4. Yeh JM, Hook EW 3rd, Golide SJ. A refined estimate of the average lifetime cost of pelvic inflammatory disease. Sex Transm Dis. 2003; 30:369–378. PMID: 12916126.

5. Yorke JA, Hethcote HW, Nold A. Dynamics and control of the transmission of gonorrhea. Sex Transm Dis. 1978; 5:51–56. PMID: 10328031.

6. Garnett GP, Bowden FJ. Epidemiology and control of curable sexually transmitted diseases: opportunities and problems. Sex Transm Dis. 2000; 27:588–599. PMID: 11099074.
7. Kretzschmar M, van Duynhoven YT, Severijnen AJ. Modeling prevention strategies for gonorrhea and Chlamydia using stochastic network simulations. Am J Epidemiol. 1996; 144:306–317. PMID: 8686700.

8. Stamm WE, Koutsky LA, Benedetti JK, Jourden JL, Brunham RC, Holmes KK. Chlamydia trachomatis urethral infections in men. Prevalence, risk factors, and clinical manifestations. Ann Intern Med. 1984; 100:47–51. PMID: 6546328.
9. Marrazzo JM, Whittington WL, Celum CL, Handsfield HH, Clark A, Cles L, et al. Urine-based screening for Chlamydia trachomatis in men attending sexually transmitted disease clinics. Sex Transm Dis. 2001; 28:219–225. PMID: 11318253.

10. Creighton S, Tenant-Flowers M, Taylor CB, Miller R, Low N. Co-infection with gonorrhoea and chlamydia: how much is there and what does it mean? Int J STD AIDS. 2003; 14:109–113. PMID: 12662389.

11. Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis control program on susceptibility to reinfection. J Infect Dis. 2005; 192:1836–1844. PMID: 16235186.
12. Barnes RC, Rompalo AM, Stamm WE. Comparison of Chlamydia trachomatis serovars causing rectal and cervical infections. J Infect Dis. 1987; 156:953–958. PMID: 3680995.

13. Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004; 36:6–10. PMID: 14982671.

14. McMillan A, Sommerville RG, McKie PM. Chlamydial infection in homosexual men: Frequency of isolation of Chlamydia trachomatis from the urethra, ano-rectum, and pharynx. Br J Vener Dis. 1981; 57:47–49. PMID: 7470835.

15. Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004; 291:2229–2236. PMID: 15138245.

16. Quinn TC, Goodell SE, Mkrtichian E, Schuffler MD, Wang SP, Stamm WE, et al. Chlamydia trachomatis proctitis. N Engl J Med. 1981; 305:195–200. PMID: 7017409.
17. Jones RB, Rabinovitch RA, Katz BP, Batteiger BE, Quinn TS, Terho P, et al. Chlamydia trachomatis in the pharynx and rectum of heterosexual patients at risk for genital infection. Ann Intern Med. 1985; 102:757–762. PMID: 3888022.

18. Lyss SB, Kamb ML, Peterman TA, Moran JS, Newman DR, Bolan G, et al. Chlamydia trachomatis among patients infected with and treated for Neisseria gonorrhoeae in sexually transmitted disease clinics in the United States. Ann Intern Med. 2003; 139:178–185. PMID: 12899585.

19. Korenromp EL, Sudaryo MK, de Vlas SJ, Gray RH, Sewankambo NK, Serwadda D, et al. What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? Int J STD AIDS. 2002; 13:91–101. PMID: 11839163.

20. Handsfield HH, Alexander ER, Pin Wang S, Pedersen AH, Holmes KK. Differences in the therapeutic response of chlamydia positive and chlamydia-negative forms of nongonococcal urethritis. J Am Vener Dis Assoc. 1976; 2:5–9. PMID: 767309.
21. Hillis SD, Coles FB, Litchfield B, Black CM, Mojica B, Schmitt K, et al. Doxycycline and azithromycin for prevention of chlamydial persistence or recurrence one month after treatment in women. A use-effectiveness study in public health settings. Sex Transm Dis. 1998; 25:5–11. PMID: 9437777.
22. Shafer MA, Schachter J, Moncada J, Keogh J, Pantell R, Gourlay L, et al. Evaluation of urine based screening strategies to detect Chlamydia trachomatis among sexually active asymptomatic young males. JAMA. 1993; 270:2065–2070. PMID: 8411573.
23. Oriel JD, Reeve P, Thomas BJ, Nicol CS. Infection with Chlamydia group A in men with urethritis due to Neisseria gonorrhoeae. J Infect Dis. 1975; 131:376–382. PMID: 804022.

24. Holmes KK, Handsfield HH, Wang SP, Wentworth BB, Turck M, Anderson JB, et al. Etiology of nongonococcal urethritis. N Engl J Med. 1975; 292:1199–1205. PMID: 165407.

25. Berger RE, Alexander ER, Harnisch JP, Paulsen CA, Monda GD, Ansell J, et al. Etiology, manifestations and therapy of acute epididymitis: Prospective study of 50 cases. J Urol. 1979; 121:750–754. PMID: 379366.

26. Kousa M, Saikku P, Richmond S, Lassus A. Frequent association of chlamydial infection with Reiter's syndrome. Sex Transm Dis. 1978; 5:57–61. PMID: 10328032.

27. Paavonen J, Stevens CE, Wølner-Hanssen P, Critchlow CW, Derouen T, Kiviat N, et al. Colposcopic manifestations of cervical and vaginal infections. Obstet Gynecol Surv. 1988; 43:373–381. PMID: 3419730.

28. Brunham RC, Paavonen J, Stevens CE, Kiviat N, Kuo CC, Critchlow CW, et al. Mucopurulent cervicitis-the ignored counterpart in women of urethritis in men. N Engl J Med. 1984; 311:1–6. PMID: 6427611.

29. Black CM. Current methods of laboratory diagnosis of Chlamydia trachomatis infections. Clin Microbiol Rev. 1997; 10:160–184. PMID: 8993862.

30. Marrazzo JM, Stamm WE. New approaches to the diagnosis, treatment, and prevention of chlamydial infection. Curr Clin Top Infect Dis. 1998; 18:37–59. PMID: 9779350.
31. Bauwens JE, Clark AM, Loeffelholz MJ, Herman SA, Stamm WE. Diagnosis of Chlamydia trachomatis urethritis in men by polymerase chain reaction assay of first-catch urine. J Clin Microbiol. 1993; 31:3013–3016. PMID: 8263188.

32. Bianchi A, Scieux C, Brunat N, Vexiau D, Kermanach M, Pezin P, et al. An evaluation of the polymerase chain reaction amplicor Chlamydia trachomatis in male urine and female urogenital specimens. Sex Transm Dis. 1994; 21:196–200. PMID: 7974069.

33. Arya OP, Hobson D, Hart CA, Bartzokas C, Pratt BC. Evaluation of ciprofloxacin 500 mg twice daily for one week in treating uncomplicated gonococcal chlamydial, and non-specific urethritis in men. Genitourin Med. 1986; 62:170–174. PMID: 2942454.

34. Hooton TM, Batteiger BE, Judson FN, Spruance SL, Stamm WE. Ofloxacin versus Doxycycline for treatment of cervical infection with Chlamydia trachomatis. Antimicrob Agents Chemother. 1992; 36:1144–1146. PMID: 1510408.

35. Ossewaarde JM, Plantema FH, Rieffe M, Nawrocki RP, de Vries A, van Loon AM. Efficacy of single-dose azithromycin versus doxycycline in the treatment of cervical infections caused by Chlamydia trachomatis. Eur J Clin Microbiol Infect Dis. 1992; 11:693–697. PMID: 1330565.
36. Bowie WR, Alexander ER, Stimson JB, Floyd JF, Holmes KK. Therapy for nongonococcal urethritis: Double-blind randomized comparison of two doses and two durations of minocycline. Ann Intern Med. 1981; 95:306–311. PMID: 7271091.
37. Prentice MJ, Taylor-Robinson D, Csonka GW. Non-specific urethritis: A placebo-controlled trial of minocycline in conjunction with laboratory investigations. Br J Vener Dis. 1976; 52:269–275. PMID: 786440.

38. Morton R. Rook A, editor. Gonorrhoea, Vol. 9 in the series Major Problems in Dermatology. 1977. London: W.B. Saunders.
39. Rosebury T. Microbes and morals: the strange story of venereal disease. 1971. New York: Viking Adult.
40. Kampmeier RH. Identification of the gonococcus by Albert Neisser. Sex Transm Dis. 1978; 5:71–72. PMID: 10328036.

41. Kampmeier RH. Introduction of sulfonamide therapy for gonorrhea. Sex Transm Dis. 1983; 10:81–84. PMID: 6362039.

42. Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women; results from a cohort study. AIDS. 1993; 7:95–102. PMID: 8442924.
43. Memish ZA, Osoba AO. Sexually transmitted diseases and travel. Int J Antimicrob Agents. 2003; 21:131–134. PMID: 12615376.

44. Fenton KA, Lowndes CM. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004; 80:255–263. PMID: 15295121.

45. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Surveillance, 2005. 2006. 11. Atlanta, GA. U.S. Department of Health and Human Services, Public Health Service.
46. 2006 World Health Organization. CISID database. Sexually transmitted infections. Accessed March 28, 2007. http://data.euro.who.int/cisid/default.aspx?TabID=123064.
47. Barnes RC, Holmes KK. Epidemiology of gonorrhea: Current perspectives. Epidemiol Rev. 1984; 6:1–30. PMID: 6436045.

48. Lafferty WE, Hughes JP, Handsfield HH. Sexually transmitted disease in men who have sex with men: Acquisition of gonorrhea and nongonococcal urethritis by fellatio and implications for STD/HIV prevention. Sex Transm Dis. 1997; 24:272–278. PMID: 9153736.
49. Kellogg DS Jr, Peacock WL Jr, Deacon WE, Brown L, Pirkle DI. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1963; 85:1274–1279. PMID: 14047217.
50. Bruins SC, Tight RR. Laboratory-acquired gonococcal conjunctivitis. JAMA. 1979; 241:274. PMID: 102807.

51. Pearce WA, Buchanan TM. Attachment role of gonococcal pili: Optimum conditions and quantitation of adherence of isolated pili to human cells in vitro. J Clin Invest. 1978; 61:931–943. PMID: 96134.

52. King GJ, Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978; 21:575–584. PMID: 211086.

53. Holmes KK, Johnson DW, Trostle HJ. An estimate of the risk of men acquiring gonorrhea by sexual contact with infected females. Am J Epidemiol. 1970; 91:170–174. PMID: 5416250.
54. Hooper RR, Reynolds GH, Jones OG, Zaidi A, Wiesner PJ, Latimer KP, et al. Cohort study of venereal disease: 1. The risk of gonorrhea transmission from infected women to men. Am J Epidemiol. 1978; 108:136–144. PMID: 707474.
56. Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med. 1974; 290:117–123. PMID: 4202519.
57. Robinson AJ, Ridgway GL. Concurrent gonococcal and chlamydial infection: how best to treat. Drugs. 2000; 59:801–813. PMID: 10804036.
58. Harrison WO, Hooper RR, Wiesner PJ, Campbell AF, Karney WW, Reynolds GH, et al. A trial of minocycline given after exposure to prevent gonorrhea. N Engl J Med. 1979; 300:1074–1078. PMID: 107450.

59. Jacobs NF, Kraus SJ. Gonococcal and nongonococcal urethritis in men: Clinical and laboratory differentiation. Ann Intern Med. 1975; 82:7–12. PMID: 67816.
60. Barlow D, Phillips I. Gonorrhea in women: Diagnostic, clinical, and laboratory aspects. Lancet. 1978; 1:761–764. PMID: 76760.
61. Platt R, Rice PA, McCormack WM. Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. JAMA. 1983; 250:3205–3209. PMID: 6417362.

62. Curran JW, Rendtorff RC, Chandler RW, Wiser WL, Robinson H. Female gonorrhea: Its relation to abnormal uterine bleeding, urinary tract symptoms, and cervicitis. Obstet Gynecol. 1975; 45:195–198. PMID: 804148.
63. Lebedeff DA, Hochman EB. Rectal gonorrhea in men: Diagnosis and treatment. Ann Intern Med. 1980; 92:463–466. PMID: 6767428.

64. Wiesner PJ, Tronca E, Bonin P, Pedersen AH, Holmes KK. Clinical spectrum of pharyngeal gonococcal infections. N Engl J Med. 1973; 288:181–185. PMID: 4264580.
65. Hutt DM, Judson FN. Epidemiology and treatment of oropharyngeal gonorrhea. Ann Intern Med. 1986; 104:655–658. PMID: 2938529.

66. Johnson RE, Newhall WJ, Papp JR, Knapp JS, Black CM, Gift TL, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections - 2002. MMWR Recomm Rep. 2002; 51:1–38. PMID: 12418541.
67. Cook RL, Hutchison SL, Østergaard L, Braithwaite RS, Ness RB. Systematic review: Noninvasive testing for chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med. 2005; 142:914–925. PMID: 15941699.

68. Koumans EH, Johnson RE, Knapp JS, St Louis ME. Laboratory testing for Neisseria gonorrhoeae by recently introduced nonculture tests: a performance review with clinical and public health considerations. Clin Infect Dis. 1998; 27:1171–1180. PMID: 9827265.
69. Davies PO, Low N, Ison CA. The role of effective diagnosis for the control of gonorrhoea in high prevalence populations. Int J STD AIDS. 1998; 9:435–443. PMID: 9702590.

70. Boslego JW, Tramont EC, Takafuji ET, Diniega BM, Mitchell BS, Small JW, et al. Effect of spectinomycin use on the prevalence of spectinomycin-resistant and of penicillinase-producing Neisseria gonorrhoeae. N Engl J Med. 1987; 317:272–278. PMID: 2955222.
71. Burstein GR, Berman SM, Blumer JL, Moran JS. Ciprofloxacin for the treatment of uncomplicated gonorrhea infection in adolescents: does the benefit outweigh the risk? Clin Infect Dis. 2002; 35(suppl 2):S191–S199. PMID: 12353206.

72. Handsfield HH, Dalu ZA, Martin DH, Douglas JM Jr, McCarty JM, Schlossberg D. Azithromyclin Gonorrhea Study Group. Multicenter trial of single-dose azithromycin vs. ceftriaxone in the treatment of uncomplicated gonorrhea. Sex Transm Dis. 1994; 21:107–111. PMID: 9071422.

73. Paster BJ, Dewhirst FE. Phylogenetic foundation of spirochetes. J Mol Microbiol Biotechnol. 2000; 2:341–344. PMID: 11075904.
74. Garnett GP, Aral SO, Hoyle DV, Cates W Jr, Anderson RM. The natural history of syphilis. Implications for the transmission dynamics and control of infection. Sex Transm Dis. 1997; 24:185–120. PMID: 9101629.
75. Klausner JD, Wolf W, Fischer-Ponce L, Zolt I, Katz MH. Tracing a syphilis outbreak through cyberspace. JAMA. 2000; 284:447–449. PMID: 10904507.

76. Ratnam S. The laboratory diagnosis of syphilis. Can J Infect Dis Med Microbiol. 2005; 16:45–51. PMID: 18159528.

77. Nandwani R, Evans DT. Are you sure it's syphilis? A review of false positive serology. Int J STD AIDS. 1995; 6:241–248. PMID: 7548285.

78. Romanowski B, Sutherland R, Fick GH, Mooney D, Love EJ. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991; 114:1005–1009. PMID: 2029095.

79. Mackay IM, Harnett G, Jeoffreys N, Bastian I, Sriprakash KS, Siebert D, et al. Detection and discrimination of herpes simplex viruses, Haemophilus ducreyi, Treponema pallidum, and Calymmatobacterium (Klebsiella) granulomatis from genital ulcers. Clin Infect Dis. 2006; 42:1431–1438. PMID: 16619156.

80. Turner TB, Hardy PH, Newman B. Infectivity tests in syphilis. Br J Vener Dis. 1969; 45:183–195. PMID: 4899592.

81. Bauer TJ, Price EV, Cutler JC. Spinal fluid examinations among patients with primary or secondary syphilis. Am J Syph. 1952; 36:309–318. PMID: 14933675.
82. Hook EW 3rd, Marra CM. Acquired syphilis in adults. N Engl J Med. 1992; 326:1060–1069. PMID: 1549153.

83. Rolfs RT, Joesoef MR, Hendershot EF, Rompalo AM, Augenbraun MH, Chiu M, et al. The Syphilis and HIV Study Group. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. N Engl J Med. 1997; 337:307–314. PMID: 9235493.

84. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003; 290:1510–1514. PMID: 13129993.
85. Brown ST. Adverse reactions in syphilis therapy. J Am Vener Dis Assoc. 1976; 3:172–176. PMID: 1010785.
86. Zhou P, Gu Z, Xu J, Wang X, Liao K. A study evaluating ceftriaxone as a treatment agent for primary and secondary syphilis in pregnancy. Sex Transm Dis. 2005; 32:495–498. PMID: 16041252.

87. Ghanem KG, Erbelding EJ, Cheng WW, Rompalo AM. Doxycycline compared with benzathine penicillin for the treatment of early syphilis. Clin Infect Dis. 2006; 42:e45–e49. PMID: 16477545.

88. Riedner G, Rusizoka M, Todd J, Maboko L, Hoelscher M, Mmbando D, et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005; 353:1236–1244. PMID: 16177249.

89. Lukehart SA. Serologic testing after therapy for syphilis: is there a test for cure? Ann Intern Med. 1991; 114:1057–1058. PMID: 2029102.

90. Ghanem KG, Erbelding EJ, Wiener ZS, Rompalo AM. Serological response to syphilis treatment in HIV-positive and HIV-negative patients attending sexually transmitted diseases clinics. Sex Transm Infect. 2007; 83:97–101. PMID: 16943224.

91. Centers for Disease Control and Prevention. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006; 55:1–94. PMID: 16888612.

92. Morse SA. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989; 2:137–157. PMID: 2650859.

93. Hammond GW, Slutchuk M, Scatliff J, Sherman E, Wilt JC, Ronald AR. Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev Infect Dis. 1980; 2:867–879. PMID: 6971469.

94. Lewis D. Chancroid: clinical manifestations, diagnosis and management. Sex Transm Infect. 2003; 79:68–71. PMID: 12576620.

95. Tyndall MW, Agoki E, Plummer FA, Malisa W, Ndinya-Achola JO, Ronald AR. Single dose azithromycin for the treatment of chancroid: a randomized comparison with erythromycin. Sex Transm Dis. 1994; 21:231–234. PMID: 7974076.
96. Martin DH, Sargent SJ, Wendel GD Jr, McCormack WM, Spier NA, Johnson RB. Comparison of azithromycin and ceftriaxone for the treatment of chancroid. Clin Infect Dis. 1995; 21:409–414. PMID: 8562752.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download