Abstract
Purpose
To investigate pathophysiological consequences and spontaneous recovery after cavernous nerve crush injury (CNCI) in a rat model.
Materials and Methods
Twenty 4-week-old male Sprague-Dawley rats were divided into the following groups: sham-operated group (n=10) and bilateral CNCI groups (n=10) for two different durations (12 and 24 weeks). At both time points, CN electrical stimulation was used to assess erectile function by measuring the intracavernous pressure. The expression of hypoxia inducible factor (HIF)-1α and sonic hedgehog (SHH) was examined in penile tissue. Immunohistochemical staining was performed for nerve growth factor (NGF), endothelial nitric oxide synthase (eNOS), neuronal nitric oxide synthase (nNOS), and smooth muscle α-actin.
Results
CNCI significantly decreased erectile function at 12 weeks (51.7% vs. 71.9%, mean ICP/BP ratio, p<0.05) and increased the expression of HIF-1α and decreased the expression of eNOS, nNOS, and SHH. At 24 weeks, erectile function in the CNCI group was improved with no significant difference versus the sham group (70.5% vs. 63.3%, mean ICP/BP ratio, p<0.05) or the CN group at 12 weeks (51.7% vs. 63.3%, mean ICP/BP ratio, p<0.05). By RT-PCR, the increase in HIF-1α and decrease in SHH mRNA was restored at 24 weeks. By immunohistochemistry, the expression of eNOS and nNOS was increased at 24 weeks.
Conclusions
CN injury induces significantly impaired erectile function and altered gene and protein expression, which suggests that local hypoxic and inflammatory processes may contribute to this change. Significant spontaneous recovery of erectile function was observed at 6 months after CN crush injury.
Neuropraxia is caused by a nerve that has been excessively strained during traction on the prostate, in an electrocautery-related heat injury, in a devascularization-related ischemic injury, or during an inflammatory response. Cavernous nerve (CN) injury is caused partially by Wallerian degeneration; specifically, nerves attached to the corpus cavernosum are blocked and as a result the corpus cavernosal smooth muscle and tunica albuginea degenerate and atrophy. In an animal study, erectile dysfunction was found to occur only by the exposure of the cavernous nerve without artificial intervention. This implies that even a minor nerve injury can affect penile erection [1]. Neuropraxia secondarily causes apoptosis in corpus cavernosal smooth muscle, a change in the ratio of smooth muscle to collagen, and the contraction of endotheliocytes and thus negatively affects the recovery of erection [2-4].
This study was conducted on 20 male Sprague-Dawley rats (DBL, South Korea) that were classified into 2 groups by tens, one to undergo sham operations (SH group) and the other to undergo surgery for their cavernous nerve crush injury (CNCI group). At 12 weeks post-injury, the experiments were performed on 5 rats in each group. Animals were raised under the same conditions before undergoing the experiments.
In the CNCI group, ketamine (100 mg/kg) and xylazine (10 mg/kg) were injected into the abdominal cavity before the surgery, and body heat was maintained by using a heating pad. The prostate was exposed, and the major pelvic ganglion lying in the dorsal prostate and the cavernous nerve were checked. A spot, which was 5 mm distant from the major pelvic ganglion, was then compressed with a curved hemostat for 30 s, 3 times, at intervals of 30 s. After the procedure was performed on both nerves in the same way, the abdominal cavity was closed. In the sham group, the surgery was performed by the same method as used in the CNCI group but without the CN crushing procedure.
To quantify erectility after neuropraxia, intracavernous pressure (ICP) and mean arterial pressure (MAP) were measured in anesthetized rats 12 and 24 weeks after the experiment. The left carotid artery was exposed to measure MAP, whereby a PE-50 tube filled with 50 IU heparinized saline was inserted. ICP was monitored through a 23 G needle inserted into the corpus cavernosum after the skin and the fascia were removed from the penis. Then, electrical stimulation was applied to the CN to induce penile erection. This electrical stimulation was performed for 60 s with a bipolar electrode at 1.5 mA, 20 Hz, and 7.5 V with a square-wave duration of 5 ms. MAP and ICP were recorded by using a polygraph. Data were processed with a signal processor, and erectile function was quantified by ICP-MAP ratio (%) (Fig. 1A).
At the 12th week and 24th week, corpus cavernosal samples were dissected, and mRNA samples were isolated for RT-PCR for the hypoxia-inducible factor (HIF)1-α and SHH mRNA. RT-PCR was performed on separated RNA at 48℃ for 45 minutes. The RNA-cDNA hybrid was degenerated by being cultured at 94℃ for 2 minutes. An access RT-PCR system (Promega, Madison, WI, USA) was used for all processes. PCR products were identified by agarose gel electrophoresis and ethidium bromide staining.
The amputated corporal tissue was measured by the use of NGF, eNOS, nNOS, and α-smooth muscle actin (SMA) antibodies by routine immunohistochemistry. Tissue samples were made to react to antibodies diluted with antibody diluent at 4℃ for 24 hours. Immunoreactive materials were identified by using avidin-biotin-peroxidase complex solution in the Vectastatin ABC kit (Vector Laboratories, Burlingame, CA, USA) by using 3,3-diaminobenzidine (Zymed Laboratories, San Francisco, CA, USA).
To quantify apoptosis in penile cavernous tissue, terminal deoxynucleotidyl transferase-medicated nick-end labeling (TUNEL) staining was performed with an apoptosis detection kit (Roche Diagnostica GmbH, Penzberg, Germany). To perform image analyses, 5 randomly selected regions from each rat were magnified 400 times, and images were analyzed by using Image-Pro Plus 4.1 software (Media Cybernetics, Bethesda, MD, USA).
At the 12th week, erectility was significantly lower in the CNCI group than in the sham group (51.7% vs. 71.9%; mean ICP-BP ratio; p<0.05). At the 24th week, however, no significant intergroup differences were observed (63.3% vs. 70.5%; mean ICP-BP ratio; p<0.05) (Fig. 1B).
The expression of HIF-1α and SHH mRNA was analyzed by RT-PCR at week 12. The CNCI group showed a significant increase in HIF-1α and a decrease in SHH mRNA compared with the sham group. At week 24, on the other hand, the increase or decrease was diminished (Fig. 2).
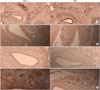
NGF, eNOS, nNOS, and α-actin were analyzed through immunohistochemistry. At the 12th week, the CNCI group showed a significant decrease in relation to the area of staining for eNOS, nNOS, and α-actin in comparison with the sham group. In relation to NGF, however, it showed an increase (Fig. 3). At the 24th week, no significant changes were observed.
Approximately 50,000 cases of nerve-sparing radical prostatectomy are performed annually in the United States. However, sexually active survivors often complain of erectile dysfunction (ED) after undergoing surgery [1]. Intraoperative nerve injury causes endothelial damage and impairs the interaction between the CN and penile tissue, which results in atrophy of the corpus cavernosum, the loss of neurotransmitters, and fibrosis. Eventually, ED may occur [6,7].
The term neuropraxia originates from the Seddon classification, according to which it is defined as temporary neuromotor paralysis, sometimes with an accompanying neurosensory or autonomic disturbance. Neuropraxia is the mildest nerve injury without rupture of nerves and neurilemmas. It may be restored without Wallerian degeneration, which blocks impulse conduction within the nerve fiber. This biochemical mechanism is made possible when the nerve fiber is injured by concussion or shock. It is inevitable that neuropraxia occurs in the process of a prostate operation no matter how careful the operator is, because the prostate and the nerve are very close to each other [1].
In the physiologic studies conducted during this study, at the 12th week, the CNCI group showed a significant decrease in erectility compared with the sham group. The ICP-MAP ratio was also lowered. According to the reports of recent studies, postoperative ED causes poor cavernosal oxygenation and corporal fibrosis, and the final outcome is that it causes permanent ED [6,8]. The decrease in penile blood flow gives rise to the apoptosis of cavernosal and endothelial cells, which precludes penile distensibility [9]. At 24 weeks afterward, no significant intergroup differences were observed in relation to erectility. These findings may be interpreted to mean that erectile function was restored during the period [10]. HIF-1α is a transcription of a gene encoding protein for controlling oxygen homeostasis. The expression of HIF-1α increased in the CNCI group, which is associated with the hypoxic state related to penile blood flow. When an organ reaches the hypoxic state due to a physiological or pathological problem, it triggers cellular reactions so as to oxygenate tissue and receive an energy source. HIF-1α is the transcription factor at the center of such reactions [11,12]. The results of RT-PCR showed that HIF-1α increased significantly at the 12th week, but at the 24th week, the CNCI group did not show a significant increase. In response to the hypoxic state, the human body increases oxygen transmission so that each cell may undergo corrective or adaptive reactions to induce oxygen homeostasis [13]. It is presumed that such mechanisms were the very reason the expression of HIF-1α decreased at the 24th week.
SHH is an essential regulator of penile smooth muscle and apoptosis that is critical for normal erectile function [14,15]. SHH is synthesized as a 45 to 49 kDa secreted precursor that undergoes autoproteolytic cleavage to yield two mature proteins: a 19-kDa amino-terminal fragment that is cholesterol modified, palmitoylated, and is biologically active, and a 25 to 31 kDa carboxy-terminal fragment that retains no known biological activity [15,16]. SHH is necessary during embryogenesis of the penis for both genital tubercle outgrowth and differentiation [17]. In mice with a targeted deletion of SHH, external genitalia are completely absent [18]. The SHH pathway functions after birth to direct differentiation of corpora cavernosal sinuses [19], and in the adult penis, SHH functions to maintain the sinusoid morphology of the corpora cavernosa that it helped to establish [19]. When SHH signaling is inhibited in the adult penis, there is a significant 12-fold increase in smooth muscle apoptosis [14], which alters sinusoidal morphology and causes ED. In this study, the expression of SHH decreased at the 12th week, but at the 24th week, the decrease tended to be restored, which might come from recovery. NGF induces the expression of NOS, whereas a product, NO, intervenes in the differentiation of nerve cell terminals. Given this fact, it is deemed that when the nerve is injured, NO interacts with neurotrophic factors to bring penile erectile tissue to functional perfection [1]. This implies that intracellular components such as eNOS and nNOS are concerned with ED through CNCI or the endothelial injury. In the immunohistologic analysis performed during this study, at the 12th week, the CNCI group showed a significant decrease in NGF, eNOS, and nNOS compared with the SH group. At the 24th week, the decrease decelerated, but it was not statistically significant.
The results of this study suggest that ED is associated with erectile dysfunction caused by CNCI-related neuropraxia, inflammatory response, and local hypoxia caused by the expression of modified genes and proteins. In addition, our results suggest that 6 months after CNCI, erectile function may be spontaneously restored to a significant degree.
Figures and Tables
FIG. 1
(A) Electrical stimulation of the cavernous nerve to induce penile erection. ▽: starting point of stimulation, ▼: maximal point of ICP/MAP (%). After stimulation, the ICP/MAP (%) of the CNCI group was lower than that of the sham group. (B) Functional results. Erectile function was measured in response to electrical stimulation of the cavernous nerve at 12 weeks or 24 weeks after CNCI. The CNCI group at 12 weeks had significantly decreased erectile dysfunction. At 24 weeks, however, the erectile function of the CNCI group was improved with no significant difference. The results are expressed as the mean±standard deviation. a: p<0.05.

FIG. 2
Reverse transcription-polymerase chain reaction (RT-PCR) amplification for hypoxia-inducible factor (HIF)-1α and sonic hedgehog (SHH) mRNA was performed after 12 weeks or 24 weeks. Cavernous nerve crush injury (CNCI) increased the expression of HIF-1α and decreased the expression of SHH. The densitometry data were normalized by b-actin and expressed as a ratio of sham values. a: p<0.05.
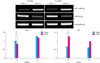
FIG. 3
Immunohistochemistry at 12 weeks. Representative slides of immunostaining of (B) nerve growth factor (NGF), (C) endothelial nitric oxide synthase (eNOS), (D) neuronal nitric oxide synthase (nNOS), and (A) α actin. Expression of NGF is increased, whereas that of the others is decreased. The images are at ×100 magnification except for actin (H&E, ×40).
References
1. Burnett AL. Rationale for cavernous nerve restorative therapy to preserve erectile function after radical prostatectomy: results from CaPSURE. Urology. 2003. 61:491–497.
2. Mulhall JP, Slovick R, Hotaling J, Aviv N, Valenzuela R, Waters WB, et al. Erectile dysfunction after radical prostatectomy: hemodynamic profiles and their correlation with the recovery of erectile function. J Urol. 2002. 167:1371–1375.
3. Mulhall J. Neuroimmunophilin ligands protect cavernous nerves after crush injury in the rat: new experimental paradigms. Eur Urol. 2007. 51:1488–1489.
4. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006. 50:711–718.
5. Angeloni NL, Bond CW, Monsivais D, Tang Y, Podlasek CA. The role of hedgehog-interacting protein in maintaining cavernous nerve integrity and adult penile morphology. J Sex Med. 2009. 6:2480–2493.
6. Bond C, Tang Y, Podlasek CA. Neural influences on sonic hedgehog and apoptosis in the rat penis. Biol Reprod. 2008. 78:947–956.
7. Podlasek CA, Gonzalez CM, Zelner DJ, Jiang HB, McKenna KE, McVary KT. Analysis of NOS isoform changes in a post radical prostatectomy model of erectile dysfunction. Int J Impot Res. 2001. 13:Suppl 5. S1–S15.
8. Jung GW, Kwak JY, Kim JK, Kim DH, Yoon JH. The action mechanism of growth hormone on regeneration of nitric oxide synthase(NOS)-containing nerves after cavernous neurotomy in the rat. Korean J Urol. 1999. 40:1043–1050.
9. Klein LT, Miller MI, Buttyan R, Raffo AJ, Burchard M, Devris G, et al. Apoptosis in the rat penis after penile denervation. J Urol. 1997. 158:626–630.
10. Chung H, Lee CK, Kim BK, Kim HS, Kim TW, Paick SH, et al. Proteomic analysis of penile protein alterations in a rat model of cavernous nerve injury. Korean J Urol. 2009. 50:498–504.
11. Moreland RB, Traish A, McMillin MA, Smith B, Goldstein I, Saenz de Tejada I. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle. J Urol. 1995. 153:826–834.
12. Chiappe-Gutierrez M, Kitzmueller E, Labudova O, Fuerst G, Hoeger H, Hardmeier R, et al. mRNA levels of the hypoxia inducible factor (HIF-1) and DNA repair genes in perinatal asphyxia of the rat. Life Sci. 1998. 63:1157–1167.
13. Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996. 16:4604–4613.
14. Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod. 2007. 76:19–28.
15. Bond C, Tang Y, Podlasek CA. Neural influences on sonic hedgehog and apoptosis in the rat penis. Biol Reprod. 2008. 78:947–956.
16. Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996. 274:255–259.
17. Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroish T, et al. Unique functions of sonic hedgehog signaling during external genitalia development. Development. 2001. 128:4241–4250.
18. Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic Hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002. 247:26–46.
19. Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, et al. Sonic hedgehog cascade is required for penile postnatal morphogenesis, differentiation, and adult homeostasis. Biol Reprod. 2003. 68:423–438.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download