Abstract
Purpose
Cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1) is a key enzyme in the androgen biosynthesis pathway. CYP17A1 has been focused on because of the promising results of a potent CYP17A1 inhibitor in the treatment of castration-resistant prostate cancer (CRPC). A hypothesis that intratumoral androgenesis may play a role in the progression of CRPC has recently been postulated. Thus, we evaluated whether commonly used prostate cancer cell lines express CYP17A1.
Materials and Methods
Androgen-sensitive LNCaP and androgen-insensitive PC-3 and DU145 cells were used. To evaluate the expression of CYP17A1 protein and RNA, we performed Western blotting and RT-PCR, respectively.
In Western countries, prostate cancer (PC) is the most commonly diagnosed cancer and represents the second leading cause of death in male cancer patients [1]. In Korea, PC is the fifth most common cancer and the most rapidly increasing cancer in men in terms of incidence [2]. In particular, PCs in Korea tend to have higher Gleason scores and to be of a more advanced stage than those found in Western countries [3]. Patients with localized PC can be cured through treatment by radical prostatectomy or radiation therapy; however, 10% to 30% of these men experience biochemical recurrence. The standard treatment for patients with advanced or metastatic PC has been androgen deprivation therapy because androgens play a vital role in the development, growth, and progression of PC [4]. Nonetheless, PC in this state is incurable; it eventually progresses to castration-resistant PC (CRPC) [5]. A second-line treatment with docetaxel chemotherapy combined with prednisone is only temporarily effective, providing a 2- or 3-month survival benefit [6,7].
Recently, abiraterone acetate, which is a potent inhibitor of cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1), has been focused on for the treatment of CRPC. CYP17A1 is a key enzyme in the androgen biosynthesis pathway, which functions in the testes and adrenal glands to catalyze the conversion of pregnenolone and progesterone into the weak androgens dehydroepiandrosterone and androstenedione, respectively [8-10]. Early clinical trials demonstrated that abiraterone acetate has a significant and sustained antitumor activity in post-docetaxel CRPC [11-14]. The theoretical mechanism is that androgen-activating ligands originating in the adrenal glands or occurring by endogenous synthesis may be activating CRPC. Much evidence indicates that CRPC can synthesize androgen from cholesterol [15]. Compared with primary prostate tumors, metastatic CRPC displays alterations in the genes encoding steroidogenic enzymes, including the up-regulated expression of CYP17A1 [16]. However, compared with the promising results from clinical trials, evidence is lacking from in vitro and in vivo studies for the molecular mechanism behind the response to abiraterone acetate.
In this study, we evaluated whether commonly used prostate cancer cell lines express CYP17A1 as a part of basic research to understand the molecular pathophysiology of prostate cancer treatments.
Human prostate cancer cell lines were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). Androgen-sensitive LNCaP and androgen-insensitive PC-3 and DU145 cells were used in the experiments. The culture medium used throughout these experiments was DMEM (GibcoBRL, USA) containing 10% heat-inactivated fetal bovine serum (FBS; Mediatech, USA), 2 mm L-glutamine, 100 units/ml penicillin, and 100 µg/ml streptomycin. Cells were grown as monolayer cultures and were maintained at 37℃ under a humidified atmosphere of 5% CO2 in air. To retain the original properties of the cells, experiments were performed with cell lines at passage numbers within the range of 5 to 40.
Cells were lysed in 500 µl of cell lysis buffer (1 M Tris-Cl, pH 7.4, 0.5 M NaCl, and 0.5 M EDTA) supplemented with the protease inhibitor pepstatin (0.7 mg/ml). Cell lysates were centrifuged at 14,000 rpm for 15 minutes at 4℃. Total protein (30 mg) was resolved on 10% SDS-polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and blocked with 5% skim milk in 1% Tween-20/TBS. The membranes were incubated with a rabbit polyclonal anti-CYP17A1 antibody (NB100-92420; Novus Biological, USA) overnight. The membranes were washed and then incubated for 1.5 hours with secondary antibodies, and protein expression was detected with an ECL developing kit.
Total RNA was extracted by using the RNeasy kit (Qiagen, Germany) according to the manufacturer's protocol. Aliquots of 1 µg of total RNA were used for complementary DNA (cDNA) synthesis with a reverse transcription system (Promega, Madison, WI, USA) and oligo (dT) in a total reaction volume of 20 µl. The primer sequences used for PCR were 5'-AGCTCGTGGCTCTCTTGCTG-3' (forward) and 5'-CGCGATGTCTAGAGTTGCCA-3' (reverse). The optimal PCR conditions were determined on the basis of the amount of amplification product in proportion to the amount of input RNA. The thermal profile was 95℃ for 5 minutes, 95℃ for 30 s, and the appropriate annealing temperature for 30 s for a total of 30 cycles, and a final extension at 72℃ for 7 minutes. The annealing temperature varied between 57℃ and 62℃. The PCR products were electrophoresed on a 1% agarose gel and were visualized by ethidium bromide staining and UV irradiation.
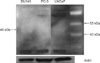
No expression of CYP17A1 was detected in any of the cell lines analyzed by Western blot despite CYP17A1 detection in a positive control cell line (HeLa cells) (Fig. 1). According to the manufacturer's protocol, the protein product is expected to be detected at approximately 48 kDa. However, we could not find any expression, even after altering the procedural conditions several times. The CYP17A1 mRNA was also not detected by RT-PCR in any of the cell lines tested (Fig. 2). The mRNA for CYP17A1 is assumed to be 307 bp in length, but we could not detect it despite several repetitions of RT-PCR. In contrast, the expression of CYP17A1 mRNA in HeLa cells was confirmed by RT-PCR.
Abiraterone acetate is a potent, selective, and irreversible inhibitor of CYP17A1 (IC50, 2-4 nmol/l) [17]. Abiraterone acetate is 10- to 30-fold more potent than the nonselective inhibitor ketoconazole. CYP17A1 plays a key role in androgen biosynthesis, functioning in both the conversion of pregnenolone to 17-α-hydroxypregnenolone (via a 17-α-hydroxylase) and the subsequent conversion of this moiety to dehydroepiandrosterone (DHEA) via a 17,20-lyase. CYP17A1 also converts progesterone to 17-α-hydroxyprogesterone and finally to androstenedione [8-10]. These androgenesis steps could be occurring in the testes and adrenal glands. DHEA and androstenedione may be converted to testosterone and consequently to dihydrotestosterone (DHT), which is a more potent androgen in peripheral tissues.
The primary concern regarding androgens and androgen receptors (ARs) in CRPC is that prostate-specific antigen (PSA) is still a prognostic surrogate in CRPC patients. The production of PSA requires activated ARs because the promoter region of the PSA gene contains androgen-responsive elements. Extensive evidence suggests that the AR plays a key role in cell proliferation in CRPC. There is an approximate 30% overexpression of the AR gene and protein after the conversion of PC to CRPC [18]. Furthermore, the sensitivity of the AR itself is much higher in CRPC cells, and the DHT level required for the proliferation of CRPC cells is approximately one quarter of that required for androgen-dependent cells [19]. Thus, the original rationale for the use of abiraterone acetate in CRPC is that a complete blockade of not only testicular but also adrenal androgen may beneficial in CRPC patients.
Furthermore, many studies of DHT in CRPC prostate tissues showed relatively maintained levels of DHT despite castration levels of serum testosterone. Mohler et al claimed that the level of androgen in CRPC prostate tissues was the same as that in normal benign hyperplastic prostate tissue, and this level was about 80% of that observed in patients who did not undergo androgen deprivation [20]. Nishiyama et al reported similar data [21]. This phenomenon has been explained by the up-regulation of 5α-reductase activity in CRPC.
A great deal of evidence has been published on endogenous androgenesis in cancer cells. Compared with primary prostate tumors, metastatic lesions of CRPC display up-regulation of genes encoding steroidogenic enzymes, including CYP17A1, FASN, HSD3B1, HSD17B3, CYP19A1, and UGT2B17. Additionally, intratumoral testosterone levels were higher than those observed in the primary tumors of eugonal patients [16]. Furthermore, recent study has demonstrated a "backdoor pathway" for the synthesis of DHT [15]. This backdoor pathway can produce DHT without requiring the steps of testosterone synthesis as an alternative to the "classic pathway" of androgenesis. In this DHT synthesis backdoor pathway, CYP17A1 also plays a major role. Thus, abiraterone acetate has been expected to block not only testicular and adrenal androgenesis but also intratumoral de novo androgen synthesis.
In clinical trials, the therapeutic effectiveness of abiraterone acetate in CRPC patients appears very promising. Phase I studies have reported that abiraterone acetate was safe and resulted in a >50% suppression of baseline testosterone in noncastrated patients [13]. Subsequently, several phase I and II studies have demonstrated that more than 50% of patients had a >50% PSA response in various settings, such as chemotherapy-naïve or post-docetaxel patients [11,12,14,22]. The encouraging data from these early experiences led to phase III studies, COU-AA-301. This trial was initiated in April of 2008 with a total of 1195 patients with docetaxel-refractory CRPC who were randomly assigned to either abiraterone acetate or placebo in a 2:1 fashion (both study arms received concomitant prednisone therapy). Following a protocol-specified interim analysis in August 2010, an independent data monitoring committee recommended that the study be unblinded because the treatment with abiraterone resulted in an improvement in overall survival from 10.9 to 14.8 months (HR 0.646, p<0.0001) [23].
In contrast to this clinical success, the exact mechanism of drug action remains unknown. Evidence from cell line or animal studies is very limited. We initially planned to prove the molecular basis of the steroidogenic and consequently oncologic effect of CYP17A1 in PC cell lines. However, we could not detect either the protein or the RNA of CYP17A1 in commonly used PC cells (LNCaP, PC-3, and DU145). This may be due to technical problems; however, it is more likely that the CYP17A1 protein and its RNA are absent in the cell lines we used or that the levels were too low to reproducibly detect. The report by Locke et al regarding the backdoor pathway showed the presence of both CYP17A1 protein and RNA in LNCaP tumors using a xenograft model [15]. Dillard et al showed the expression of CYP17A1 protein and RNA in PC-3 and DU145 cells. They also demonstrated that LNCaP cells at passage numbers less than 33 (LNCaP-C33) did not express CYP17A1, whereas cells at passage numbers over 80 (LNCaP-C81) expressed CYP17A1 [24]. LNCaP-C33 is considered to be an androgen-dependent cell line, whereas LNCaP-C81 is considered to be androgen-independent. In contrast, Hofland et al argued that the majority of tumor samples showed low or absent mRNA expression for steroidogenic enzymes required for de novo steroid synthesis [25]. Simultaneous but low expression of the enzymes CYP17A1 and 3β-hydroxysteroid dehydrogenase (HSD3B1), which are essential for the synthesis of androgens, could be detected in 19 of 88 patient samples. Of the 19 CRPC tissues examined, only 5 samples expressed both enzymes. Additionally, the expression of both enzymes was only detectable at very low levels in the two androgen-responsive cell lines (VCaP and DuCaP) and in only 2 out of 13 androgen-dependent xenografts. They also could not detect CYP17A1 RNA in LNCaP or PC346C cells. These authors concluded that intratumoral steroid biosynthesis contributes less than circulating adrenal androgens, implying that blocking androgen production and its intraprostatic conversion into DHT, such as via CYP17A1 inhibition, may represent favorable therapeutic options in patients with CRPC. The results of our study support Hofland's argument. No expression of CYP17A1 protein or mRNA was detected in any of the original cell lines tested. Thus, in our opinion, CYP17A1 expression in PC cell lines might be absent or extremely low, and it may not be reproducibly detectable and will likely have little clinical implication.
In conclusion, the expression of CYP17A1 protein and RNA in LNCaP, PC-3, and DU145 PC cell lines appears to be absent or too low to easily detect. Consequently, the mechanism of action of abiraterone acetate, a CYP17A1 inhibitor, may be related more to adrenal androgen blockade than to the blockade of intratumoral androgenesis.
Figures and Tables
Notes
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.
2. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009. 41:122–131.
3. Song C, Kang T, Lee MS, Ro JY, Lee SE, Lee E, et al. Clinico-pathological characteristics of prostate cancer in Korean men and nomograms for the prediction of the pathological stage of the clinically localized prostate cancer: a multi-institutional update. Korean J Urol. 2007. 48:125–130.
4. Goktas S, Crawford ED. Optimal hormonal therapy for advanced prostatic carcinoma. Semin Oncol. 1999. 26:162–173.
5. Gittes RF. Carcinoma of the prostate. N Engl J Med. 1991. 324:236–245.
6. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004. 351:1502–1512.
7. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004. 351:1513–1520.
8. Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005. 96:1241–1246.
9. Miller WL, Auchus RJ, Geller DH. The regulation of 17,20 lyase activity. Steroids. 1997. 62:133–142.
10. Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Semin Reprod Med. 2004. 22:281–288.
11. Attard G, Reid AH, A'Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009. 27:3742–3748.
12. Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008. 26:4563–4571.
13. O'Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004. 90:2317–2325.
14. Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010. 28:1489–1495.
15. Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008. 68:6407–6415.
16. Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008. 68:4447–4454.
17. Rowlands MG, Barrie SE, Chan F, Houghton J, Jarman M, McCague R, et al. Esters of 3-pyridylacetic acid that combine potent inhibition of 17 alpha-hydroxylase/C17,20-lyase (cytochrome P45017 alpha) with resistance to esterase hydrolysis. J Med Chem. 1995. 38:4191–4197.
18. Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997. 57:314–319.
19. Gregory CW, Johnson RT Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001. 61:2892–2898.
20. Mohler JL, Gregory CW, Ford OH 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004. 10:440–448.
21. Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004. 10:7121–7126.
22. Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010. 28:1496–1501.
23. De Bono JS, Logothetis CJ, Fizazi K, North S, Chu L, Chi KN, et al. Abiraterone acetate (AA) plus low dose prednisone (P) improves overall survival (OS) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) who have progressed after docetaxel-based chemotherapy (chemo): results of COU-AA-301, a randomized, double-blind, placebo-controlled phase III study. The 35th European Society for Medical Oncology (ESMO) annual meeting 2010. Annals of Oncology. 2010. 21:suppl 8. viii3.
24. Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008. 295:115–120.
25. Hofland J, van Weerden WM, Dits NF, Steenbergen J, van Leenders GJ, Jenster G, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010. 70:1256–1264.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download