Abstract
Purpose
Preservation of renal function is of paramount importance in patients with tumors in solitary kidneys. We compared the renal function and oncologic outcomes of patients treated by partial nephrectomy with those of patients treated by cryoablation for solitary kidney tumors.
Materials and Methods
All patients with solitary kidneys who were treated for renal tumors at our institution between 1997 and 2007 were included in the screen. We retrospectively identified 23 patients who underwent cryoablation and 15 patients who underwent partial nephrectomy.
Results
The two groups were similar with regard to age, gender, and tumor laterality. Patients in the partial nephrectomy group had a larger tumor size (3.4 cm vs. 2.5 cm, p=0.01), higher mean estimated blood loss (316 cc vs. 87 cc, p<0.001), longer duration of hospital stay (5.8 vs. 1.8 days, p<0.001), and a higher rate of perioperative complications (53.3% vs. 8.7% patients, p=0.03). Percentage changes in the glomerular filtration rate postoperatively and on follow-up were found to be similar in the two groups. Both the cryoablation and the partial nephrectomy groups with mean follow-ups of 31.2 months and 30.8 months, respectively, had evidence of local or distant recurrence in 3 patients each (13% and 20% respectively, p=0.7). Both groups had a similar mean overall survival (88.9 and 86.9 months in the cryoablation and partial nephrectomy groups, respectively, p=0.8).
A surgically resectable tumor in an anatomically or functionally solitary kidney is an absolute indication for nephron-sparing surgery (NSS) [1]. The aim of NSS is to maximize local tumor extirpation with optimal preservation of renal function to prevent the development of end-stage renal disease. Partial nephrectomy (PN) remains the treatment of choice, and encouraging oncologic outcomes in solitary kidney patients have been reported by several groups [1-3]. However, because it requires renal hilar clamping with possible renal ischemia, PN can have deleterious effects on renal function. Cryoablation offers nephron-sparing management of renal masses with fewer technical challenges, favorable oncologic results, shorter convalescence, and minimal parenchymal loss [4-6]. The feasibility and efficacy of cryoablative therapy for the management of tumors in solitary kidney patients has been reported earlier [7]. However, sufficient data are lacking comparing the impact on renal function and oncologic outcomes of the two procedures in this specific subset of patients. In this article, we present the experience of a single institution with patients undergoing cryoablation and compare renal functional outcomes with those who underwent PN for such tumors. The data were collected retrospectively and analyzed.
After obtaining the approval of the Institutional Review Board, we examined the medical records between 1997 and 2007 of all patients who underwent NSS for small, localized renal masses in a solitary kidney. The choice of procedure (cryoablation or PN) and the approach (open, laparoscopic, or percutaneous) were chosen on the basis of tumor characteristics, co-morbid conditions, patient preference, and surgeon discretion. The technique used for cryoablation has been described earlier by many authors [8-10]. An intraoperative ultrasound was used for laparoscopic and open cryoablation for tumor confirmation and monitoring of ice ball formation. Percutaneous cryoablation was done under computed tomography (CT) guidance. Both open and laparoscopic partial nephrectomy (OPN and LPN) were also performed by using standard techniques as described previously [11,12].
The clinical features studied included patient demographics, perioperative details, and follow-up data. Radiology and pathology reports were reviewed for tumor size, location, and histological subtype. Comorbidities were compared by using the Charlson Comorbidity Index [13]. Renal function was assessed by calculating the glomerular filtration rate (GFR) from serum creatinine (sCr) values by using the modification of diet in renal disease (MDRD) formula [14]. Chronic kidney disease (CKD) was defined as a GFR of less than 60 ml/min/1.73 m2. De novo CKD developed when the baseline renal function was normal but postsurgical GFR was abnormal on two separate occasions. All complications that occurred during the surgery or up to 30 days after the surgery were identified from the patients' medical records and evaluated. The patients were periodically followed up to look for any evidence of recurrence on CT imaging.
Statistical analysis was done by using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics are presented as the mean±SD and percentages. Mann-Whitney U tests were used to compare means and Fisher's exact test was used to compare proportions. A p-value of less than 0.05 was considered statistically significant. Kaplan Meier survival curves were drawn for each group, and the log rank test was used to test for differences in survival.
Of the 38 patients with a renal mass in their solitary kidney, 23 underwent cryoablation and 15 underwent PN. The patient demographics, tumor characteristics, and perioperative details are listed in Table 1. No significant differences between the two groups were found with respect to age, gender, and tumor laterality. However, patients who underwent PN had a higher mean tumor size than did the cryoablation group (3.4 cm vs. 2.5 cm, p=0.01). The mean estimated blood loss (EBL) during the procedure was also significantly higher in the PN group (316 cc vs. 87 cc respectively, p<0.001). Blood transfusion was not required in any patient who underwent cryoablation and was required in only 1 patient undergoing PN. Patients who underwent PN had a longer mean duration of hospital stay (5.8 days vs. 1.8 days, p<0.001) and a higher rate of perioperative complications (53.3% and 8.7% patients, respectively, p=0.03). Two patients (13.3%) in the PN group reported more than one complication.
In the comparison of the effect on the GFR (as shown in Table 2), there was a trend toward a better preserved GFR in the cryoablation group; however, this effect did not reach statistical significance between the two groups for percentage changes in the values on postoperative day 1 (p=0.1) or on follow-up (p=0.07). Chronic kidney disease was seen in 15 patients (65.2%) in the cryoablation group and in 9 patients (60%) in the PN group at the time of surgery. Table 3 outlines the morbidities associated with CKD, including hypertension, hyperlipidemia, diabetes, and heart disease. De novo development of CKD was seen in 3 of 8 patients (37.5%) in the cryoablation group and 2 of 6 patients (33.3%) in the PN group.
The patients were followed up for a mean period of 31.2 months in the cryoablation group and 30.8 months in the PN group. No statistical difference was observed in the rates of progression of disease, which was seen in 3 patients each (13% and 20% in the cryoablation and PN groups, respectively, p=0.7). The mean tumor size in patients who showed disease progression on follow-up was 2.1 cm in the cryoablation group and 4.5 cm in the PN group. In the cryoablation group, 2 of the patients developed distant metastases to the pancreas and the lungs. One of these patients received therapy with IL-2 and Temsirolimus, but ultimately succumbed to the disease 2 years later. The second patient was put on Sunitinib therapy, but was lost to follow-up. The third patient had a local recurrence of the tumor at the cryoablation site and underwent a radical nephrectomy. This patient required dialysis thereafter. Local recurrence of disease occurred in 2 patients in the PN group, both of whom were treated with subsequent cryoablation. The third patient had multiple metastatic pulmonary nodules and died shortly thereafter following a rapid deterioration of pulmonary function. Hemodialysis was required in 2 patients (13.3%) in this group after the procedure. One of them developed end-stage renal disease immediately after PN on removal of a large tumor burden. The second patient required hemodialysis after a nephroureterectomy done for a transitional cell carcinoma 1 year after the initial procedure. The estimated mean overall survival of 88.9 months in the cryoablation group was similar to the 86.9 months in the PN group (p=0.8 on log rank test) (Fig. 1).
Tumors in a solitary kidney represent an oncologic challenge. Partial nephrectomy is the first-line option for the management of such tumors. Numerous studies have shown promising cancer-specific outcomes along with adequate preservation of renal function in solitary kidney tumors treated with PN [1-3]. LPN and OPN were shown to be similar with regard to oncologic efficacy and renal functional outcomes in a multicenter study [15]. The duration of renal ischemia required during PN is the single most important modifiable risk factor for renal function maintenance [16]. The impact of ischemia and subsequent reperfusion injury has been studied by some groups. Beri et al used a combination of creatinine clearance time, peak concentration time, and MAG 3 isotope clearance to accurately evaluate postoperative parenchymal function [17]. Another group used the duration of renal parenchymal retention of MAG 3 as a tool to assess ischemic renal damage [18]. It is in keeping with these concerns that PN without ischemia has been gaining popularity in recent years [19,20].
Cryoablation, which completely avoids hilar clamping and the subsequent renal ischemic insult, is indicated in patients who are elderly, in patients who are poor surgical candidates owing to comorbidities or previous surgical history, and in patients with solitary kidneys [9]. Many studies have shown that cryoablation is technically less challenging, has a shorter duration of hospital stay, and is associated with improved convalescence [4-6]. Therefore, cryoablation is now being offered to many patients with cortical tumors less than 3 cm in size [6,21]. However, there is a dearth of literature comparing preservation of renal function and oncologic outcomes between PN and cryoablation in the specific cohort of patients with solitary kidney tumors.
Because a solitary kidney is the most significant risk factor for acute renal failure after NSS [22], preservation of renal function assumes a very important role while choosing the preferred mode of treatment. Deteriorating renal function is associated with an increased risk of cardiovascular morbidity, hospital admissions, and mortality [23]. We used the MDRD formula to estimate renal function. Other equations like the Cockcroft-Gault and CKD-EPI have also been shown to accurately estimate the GFR [24-26]. In two different studies, Desai et al and O'Malley et al compared the percentage changes in renal function between PN and cryoablation and found that they were similar [27,28]. A study by Turna et al showed that in solitary kidneys, the decrease in GFR caused by PN was significantly more than that caused by ablative techniques [29]. Compared with this, we found that the percentage change in the GFR postoperatively and on follow-up was not statistically different in the two groups. Although the mean values indicate a trend toward lesser impact on the GFR for cryoablation, the p-value (0.07) did not reach significance, likely representing inadequate power to detect a difference with such small sample sizes. At the time of the last follow-up after surgery, 3 patients from the cryoablation cohort (37.5%) and 2 patients from the PN group (33.3%) had developed CKD de novo. However, these patients had a relatively lower GFR at the time of surgery than did the patients who had a normal GFR on follow-up (65 vs. 78 ml/min/1.73 m2). In the follow-up of patients who had CKD at the time of treatment, only one cryoablation patient developed additional complications of CKD in the form of hypertension and diabetes, whereas two patients from the PN group had progression, one in the form of coronary artery disease with hyperlipidemia and another in the form of hypertension.
All complications that occurred during surgery or up to 30 days after the procedure were defined as perioperative complications. With the widespread use of cryoablation, we are witnessing the emergence of many complications that are specific to cryoablation [30]. Two complications in the form of atrial flutter (n=1) and sinus tachycardia (n=1) occurred in the cryoablation patients. In the PN group, eight patients developed 10 perioperative complications. These included cardiac complications (atrial flutter [n=2], myocardial infarction [n=1]), respiratory complications (pulmonary edema [n=1], pneumonia [n=1]), ureteral transection (n=1), wound infection (n=1), flank cellulitis (n=1), jaundice (n=1), and bullous impetigo (n=1). Ghavamian et al and Saranchuk et al reported complication rates of 23.8% and 26%, respectively, in patients undergoing PN for solitary kidney tumors [1,2]. We observed a relatively higher complication rate in the PN group, with 53.3% of the patients having at least one complication. By comparison with the cryoablation group (8.7%), complications in patients treated with PN were found to be significantly higher (p=0.03). Our results are in line with the data published by Desai et al, who reported a higher incidence of complications in the PN group than in the cryoablation group [27]. Turna et al published similar results with as many as 26 perioperative complications in 36 patients undergoing PN as compared with 5 complications in the cryoablation group [29]. We also found that the PN group had more blood loss and a longer duration of hospital stay than did the cryoablation group. Thus, cryoablation offers the option of a nephron-sparing modality with lower morbidity, which could be of particular importance in older patients who are considered to be poor surgical candidates [30].
Meticulous radiological studies were done at regular intervals postoperatively to look for any evidence of local or distant recurrence for an average follow-up of 30 months. Recurrence was seen in 3 patients each (13.3% and 20% in the cryoablation and PN groups, respectively). However, we found no statistically significant difference in disease recurrence or overall survival between the two groups. The three patients who witnessed a progression of disease in the PN cohort had a relatively larger mean tumor size of 4.5 cm as compared to their group (PN) mean size of 3.5 cm. The tumors in these patients were located near the hilum, making complete extirpation especially difficult. Intraoperatively, these three patients experienced a relatively higher amount of EBL (mean 1,400 cc) compared with the group's mean EBL of 316 cc. It is quite feasible that the large tumor size and the relatively inaccessible location of these tumors contributed to the higher intraoperative blood loss and an incomplete tumor excision. In the cryoablation cohort, the three patients who experienced disease recurrence had a mean tumor size of 2.1 cm, which was smaller than the group's mean tumor size of 2.5 cm. However, it is noteworthy that the tumors in these patients were also located near the hilum and were endophytic, located deep inside the kidney, making a thorough ablation relatively challenging. Thus, tumor location might have contributed to disease recurrence in these patients also.
Collectively, we have presented a comparative analysis of renal function and oncologic status after PN or cryoablation in this uncommon cohort of patients with solitary kidney tumors. Our study demonstrates that in appropriately selected patients, renal cryoablation has distinct advantages over PN, including a shorter hospital stay, a lower complication rate, and less blood loss. Also, cryoablation was successful in maintaining optimal renal function and had oncologic outcomes comparable to PN on intermediate follow-up. Thus, cryoablation offers the option of a nephron-sparing modality with impressive renal function and oncologic outcomes and lower morbidity.
We acknowledge the limitations of our study, which include among other things, the disparate tumor and sample sizes in the two groups. This could have been due to an inherent selection bias associated with the retrospective, nonrandomized design of our study. Older patients with comorbidities are considered poor surgical candidates. In these patients, cryoablation is preferred over PN to avoid the renal ischemic insult conferred by PN. Our study was also limited by its relatively small sample size and an intermediate follow-up interval, which precludes a more comprehensive multivariate statistical analysis. However, tumors in a solitary kidney are a relatively infrequent occurrence. This is also the reason the effect of the different approaches (open, laparoscopic, and percutaneous) in the two groups could not be accounted for in the analysis. The current prevailing urologic literature does not include enough parallel studies. Therefore, pooled analyses from additional reports comprising larger datasets with longer clinical follow-ups (5 and 10 years) are warranted to better delineate the role of these two procedures in maintaining renal function in this high-risk cohort.
In conclusion, the increasing frequency of detection of tumors in solitary kidneys demands a better outlined strategy with stricter selection criteria for effective treatment choice along with long-term renal function maintenance. Both cryoablation and PN are viable techniques with acceptable preservation of renal function for tumors of a small renal mass. Although PN remains the standard of care, cryoablation offers an excellent treatment alternative, with less morbidity, comparable oncologic and functional outcomes, and faster recovery.
Figures and Tables
FIG. 1
Kaplan-Meier estimated mean overall survival curves after cryoablation and partial nephrectomy in patients with tumors in solitary kidneys (log rank test p=0.8).

TABLE 1
Patient demographics, tumor characteristics, and perioperative data

CAD: coronary artery disease, CHF: congestive heart failure, CKD: chronic kidney disease, GFR: glomerular filtration rate, a: calculated by using the parameters defined in the original publication [13]
TABLE 2
Renal functional outcomes after nephron-sparing surgery
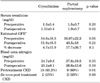
CKD: Chronic kidney disease, a: glomerular filtration rate (GFR) in ml per minute per 1.73 m2 estimated using modification of diet in renal disease (MDRD) equation [15]
References
1. Ghavamian R, Cheville JC, Lohse CM, Weaver AL, Zincke H, Blute ML. Renal cell carcinoma in the solitary kidney: an analysis of complications and outcome after nephron sparing surgery. J Urol. 2002. 168:454–459.
2. Saranchuk JW, Touijer AK, Hakimian P, Snyder ME, Russo P. Partial nephrectomy for patients with a solitary kidney: the Memorial Sloan-Kettering experience. BJU Int. 2004. 94:1323–1328.
3. Fergany AF, Saad IR, Woo L, Novick AC. Open partial nephrectomy for tumor in a solitary kidney: experience with 400 cases. J Urol. 2006. 175:1630–1633.
4. Davol PE, Fulmer BR, Rukstalis DB. Long-term results of cryoablation for renal cancer and complex renal masses. Urology. 2006. 68:1 Suppl. 2–6.
5. Aron M, Kamoi K, Remer E, Berger A, Desai M, Gill I. Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. J Urol. 2010. 183:889–895.
6. Mues AC, Landman J. Results of kidney tumor cryoablation: renal function preservation and oncologic efficacy. World J Urol. 2010. 28:565–570.
7. Shingleton WB, Sewell PE Jr. Cryoablation of renal tumours in patients with solitary kidneys. BJU Int. 2003. 92:237–239.
8. Gill IS, Novick AC, Soble JJ, Sung GT, Remer EM, Hale J, et al. Laparoscopic renal cryoablation: initial clinical series. Urology. 1998. 52:543–551.
9. Uchida M, Imaide Y, Sugimoto K, Uehara H, Watanabe H. Percutaneous cryosurgery for renal tumours. Br J Urol. 1995. 75:132–136.
10. Delworth MG, Pisters LL, Fornage BD, von Eschenbach AC. Cryotherapy for renal cell carcinoma and angiomyolipoma. J Urol. 1996. 155:252–254.
11. Novick AC. Nephron-sparing surgery for renal cell carcinoma. Annu Rev Med. 2002. 53:393–407.
12. Gill IS, Desai MM, Kaouk JH, Meraney AM, Murphy DP, Sung GT, et al. Laparoscopic partial nephrectomy for renal tumor: duplicating open surgical techniques. J Urol. 2002. 167:469–467.
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987. 40:373–383.
14. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate Glomerular filtration rate from serum creatinine. A new prediction equation. Ann Intern Med. 1999. 130:461–470.
15. Park H, Byun SS, Kim HH, Lee SB, Kwon TG, Jeon SH, et al. Comparison of laparoscopic and open partial nephrectomies in T1a renal cell carcinoma: a Korean multicenter experience. Korean J Urol. 2010. 51:467–471.
16. Lane BR, Babineau DC, Poggio ED, Weight CJ, Larson BT, Gill IS, et al. Factors predicting renal functional outcome after partial nephrectomy. J Urol. 2008. 180:2363–2368.
17. Beri A, Lattouf JB, Deambros O, Grüll M, Gschwendtner M, Ziegerhofer J, et al. Partial nephrectomy using renal artery perfusion for cold ischemia: functional and oncologic outcomes. J Endourol. 2008. 22:1285–1290.
18. Inoue Y, Kurimoto S, Kameyama S, Ohta N, Akahane M, Yoshikawa K, et al. Prolonged renal parenchymal retention of 99mTc mercaptoacetyltriglycine after nephron-sparing surgery. Nucl Med Commun. 2004. 25:509–513.
19. Janetschek G, Daffner P, Peschel R, Bartsch G. Laparoscopic nephron sparing surgery for small renal cell carcinoma. J Urol. 1998. 159:1152–1155.
20. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Comparison of warm ischemia vs. no ischemia during partial nephrectomy on a solitary kidney. Eur Urol. 2010. 58:331–336.
21. Ham BK, Kang SG, Choi H, Ko YH, Kang SH, Cheon J. The impact of renal tumor size on the efficacy of laparoscopic renal cryoablation. Korean J Urol. 2010. 51:171–177.
22. Campbell SC, Novick AC. Surgical technique and morbidity of elective partial nephrectomy. Semin Urol Oncol. 1995. 13:281–287.
23. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004. 351:1296–1305.
24. Poggio ED, Nef PC, Wang X, Greene T, Van Lente F, Dennis VW, et al. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill-hospitalized patients. Am J Kidney Dis. 2005. 46:242–252.
25. Levey AS, Lesley AS, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009. 150:604–612.
26. Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010. 55:648–659.
27. Desai MM, Aron M, Gill IS. Laparoscopic partial nephrectomy versus laparoscopic cryoablation for the small renal tumor. Urology. 2005. 66:5 Suppl. 23–28.
28. O'Malley RL, Berger AD, Kanofsky JA, Phillips CK, Stifelman M, Taneja SS. A matched-cohort comparison of laparoscopic cryoablation and laparoscopic partial nephrectomy for treating renal masses. BJU Int. 2007. 99:395–398.
29. Turna B, Kaouk JH, Frota R, Stein RJ, Kamoi K, Gill IS, et al. Minimally invasive nephron sparing management for renal tumors in solitary kidneys. J Urol. 2009. 182:2150–2157.
30. Sidana A, Agarwal P, Feng Z, Georgiades CS, Trock BJ, Rodriguez R. Complications of renal cryoablation: a single center experience. J Urol. 2010. 184:42–47.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download