Abstract
The standard treatment for a small mass has shifted from radical nephrectomy to partial nephrectomy. The benefits of partial nephrectomy, including preserving renal function, prolonging overall survival, preventing postoperative chronic kidney disease, and reducing cardiovascular events, have been discussed in many studies. With the accumulation of surgeons' experience and simplification of the operative procedures, the warm ischemic time has become shorter despite the indication of tumor size becoming larger. With the help of intraoperative ultrasound, partial nephrectomy can be performed for an endophytic renal mass. Recently, laparoscopic partial nephrectomy has become well indicated for most renal tumors in many centers with advanced laparoscopic expertise. Open partial nephrectomy remains indicated for complex tumors. With technical innovation, robotic partial nephrectomy shows at least comparable perioperative outcomes with a benefit for challenging cases. Laparoendoscopic single-site partial nephrectomy has recently been tried in limited indications and seems to be feasible.
Over 200,000 new cases of kidney cancer are diagnosed in the world each year [1]. The number of cases has been growing in Korea as well as in Europe and in the United States. According to a report of the Korea National Statistical Office, 2,846 new cases occurred in 2007.
The increase in kidney cancer may be attributed to the development of imaging modalities, which allow early detection of the tumor. Generally, these incidentally detected tumors tend to be smaller and of a lower stage. The standard treatment for a small mass has shifted from radical nephrectomy (RN) to partial nephrectomy (PN). The advantage of PN is not only excellent oncologic outcome but also better long-term preservation of renal function, leading to better overall survival.
With the pursuing of minimally invasive techniques and the rapid evolution of laparoscopic techniques, the surgical modality of PN has expanded to laparoscopic partial nephrectomy (LPN) with low overall morbidity, faster postoperative recovery [2], and comparable oncological outcomes [3]. With the widespread adoption of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA), robotic partial nephrectomy (RPN) is rapidly emerging as an alternative to traditional LPN. Recently, laparoendoscopic single-site (LESS) PN was introduced.
The objective of this article was to review the efficacy of PN by several modalities and to analyze the current role of PN.
Historically, when RN was the standard treatment for kidney cancer, PN was performed only in the patient who had a tumor in a solitary kidney, a bilateral renal tumor, or renal function impairment [4,5]. The main purpose of PN for these patients was the preserving of renal function, even though it was not enough. PN for the patient with a normal contralateral kidney is based on the concept that it provides an advantage over RN in preventing chronic kidney disease (CKD) by preserving more nephron capital. The relationship between nephron mass reduction and accelerated kidney damage was reported in experimental models [6,7]. The clinical data also indicated that the independent predictor of postoperative renal function after PN was the amount of renal volume reduction or the percentage of resected renal parenchyma [8-10].
Patients with renal cell carcinoma (RCC) may have a risk for CKD because of the shared risk of hypertension, smoking, diabetes, and advancing age [11,12]. Leaving this relation aside, recent studies reported a higher incidence of CKD after RN than after PN. A study by the MSKCC group of renal cortical tumors (≤4 cm) demonstrated that the 3-year probability of freedom of new-onset mild CKD [glomerular filtration rate (GFR) <60 ml/min/1.73 m2] was 80% after PN and 35% after RN, and that of moderate CKD (GFR <45 ml/min/1.73 m2) was 95% after PN and 64% after RN. RN was an independent risk factor for patients developing new onset of mild and moderate CKD in the multivariable analysis (hazard ratio [HR]: 3.82; 95% CI: 2.75-5.32; p<0.0001) [13].
CKD is defined as either kidney damage for ≥3 months as confirmed by structural or functional abnormalities manifested by pathologic abnormalities or markers of kidney damage with or without decreased GFR, or a decrease in GFR <60 ml/min/1.73 m2 for ≥3 months with or without kidney damage [14]. According to one study, CKD is an independent risk factor for the development of cardiovascular events, hospitalization, and death [15].
Comparing RN with PN concerning the association with an increase in cardiovascular events and mortality for patients over 66 years of age, RN was associated with an increased risk of overall mortality (HR: 1.38, p<0.01) and a 1.4 times greater number of cardiovascular events after surgery (p<0.05), although RN was not significantly associated with cardiovascular death (HR 0.95, p=0.84) [8]. Another study reported that PN had fewer adverse renal outcomes (16.4% vs. 21.8%; adjusted HR: 0.74; 95% CI: 0.58-0.94), including a trend toward less frequent receipt of dialysis services, dialysis access surgery, or renal transplantation than with RN. However, the likelihood of adverse cardiovascular outcomes did not differ by treatment [9].
The overall survival rate of RN is regarded to be lower than that of PN. A matched control study for age, year of surgery, tumor size, and Furman grade to assess the effect of nephrectomy type (RN vs. PN) on overall mortality showed that RN was associated with 1.19-fold (p=0.048) higher overall mortality rate [16]. Additionally, RN was significantly associated with death from any cause compared with PN, especially in patients under 65 years of age (relative risk: 2.16, p=0.02) (Table 1) [10].
The above studies suggest that PN is preferred for solitary small RCC or suspicious RCC. However, because these studies were retrospectively designed, there are some limitations. Selection bias in deciding on the surgery type could exist. The methods of measuring preoperative and postoperative renal impairment were diverse [estimated GFR (eGFR), serum creatinine, proteinuria, and event of visiting dialysis center]. A randomized controlled trial is required.
As imaging check-ups have become widespread, the incidence of benign tumors confirmed during the operation that had been suspected as RCC is also increasing. Despite thorough preoperative radiological evaluation, 11% to 25% of presumed RCC were revealed to be benign in recent studies [17,18]. No imaging feature available currently can accurately distinguish oncocytoma and small or lipid-poor angiomyolipoma from RCC. For these patients, PN is preferred to avoid useless nephrectomy and to preserve renal function.
Initially, PN was only accepted as the standard treatment of localized RCC in imperative or absolute indications (patients with an anatomically or functionally solitary kidney or with bilateral RCC). Relative indications were those in which a functioning contralateral kidney was affected by comorbidities that might impair renal function in the future, such as arterial hypertension, arteriosclerosis, and diabetes, including the hereditary forms of RCC. Elective indications were those in which the contralateral kidney was perfectly normal [19]. In the early days, elective PNs were performed restrictively in small, exophytic, and easily accessible tumors. Several articles reported long-term cancer-free survival rates of 92% to 100%. Therefore, PN became one of the standard treatments for patients with clinically localized RCC <4 cm (T1a tumors) in the 2007 RCC guidelines of the European Association of Urology [20].
As surgeons' experience has accumulated, attempts for larger, endophytic, and not easily accessible tumors have emerged. In a study of T1b RCC, PN and RN showed similar results for overall survival (92.3% vs. 87.8%, p=0.501), recurrent-free survival (92.3% vs. 77.8%, p=0.175), and cancer-specific survival (92.3% vs. 94.5%, p=0.936), respectively. Additionally, the proportion of patients with decreased renal function (PN=0% vs. RN=11.5%) and postoperative changes in the serum creatinine level 1 year after nephrectomy (0.2±0.2 mg/dl vs. 0.3±0.5 mg/dl, p=0.150) was better in the PN group than in the RN group [21]. In another study of T1b tumors, RN was associated with postoperative CKD in the multivariate analysis (odds ratio: 3.4; 95% CI: 2.1-5.6). The survival analysis demonstrated that PN was associated with better overall survival (HR: 0.30; 95% CI: 0.13-0.71; p=0.006) [22]. In the laparoscopic approach, compared with LRN (n=75) and LPN (n=35) for T1b-T3N0M0 RCC, overall mortality (11% vs. 11%), cancer-specific mortality (3% vs. 3%), and recurrence (3% vs. 6%) rates (p=0.4) were equivalent. The postoperative decrease in the eGFR was less in the LPN group than in the LRN group at 13 and 24 ml/min, respectively (p=0.03). Postoperatively, a two-stage increase in CKD stage occurred in 12% vs. 0% of patients in the LRN and LPN groups, respectively (p<0.001). The data suggest that LPN for a large mass provides equivalent oncologic efficacy and superior renal functional outcomes compared with LRN [23].
Concerning tumor location, Shikanov et al prospectively compared the outcomes of LPN for technically challenging tumors (endophytic tumors, tumors located near the hilum or the posterior upper pole) and tumors in other locations [24]. LPN for challenging tumors resulted in a higher rate of collecting system repair (78% vs. 61%, p=0.03). However, operative [surgery time, warm ischemia time (WIT), blood loss, and intraoperative complications] and postoperative (renal function, nadir hematocrit, complication rate, hospital stay, and positive margin rate) outcomes were similar between the groups. Therefore, with developing experience, LPN can be safe for technically challenging renal tumors in well-selected patients.
Different surgical techniques can be used to perform PN, but all of them require adherence to basic principles of early vascular control, avoidance of ischemic renal damage with complete tumor excision with free margins, precise closure of the collecting system, careful hemostasis, and closure with or without tamponading of the renal defect with adjacent fat, fascia, or any available artificial sealant [25,26]. Surgeons performing this approach require an understanding of renal responses to warm ischemia (WI) and available methods of protecting the kidney when the period of arterial occlusion exceeds normal parenchyma tolerability [27]. Although the historically safe duration of WIT from an experimental study, where full recovery of renal function is expected, is commonly thought to be 30 minutes, there are several limitations regarding functional data from the current literature [25]. Additionally, most functional study data were based on serum creatinine reporting that PN does not have an effect on renal function [28,29], which is a limited biomarker for GFR. Unfortunately, there have been significant variations in the impact of WI on renal function owing to various study protocols, possibly involved in the renal function, including solitary kidney, pneumoperitoneal effect, estimation methods of renal function, and preoperative renal function [28,30]. Furthermore, few studies in the PN literature have successfully been able to measure functional compensation by a normal contralateral kidney or a functional decrease specifically in the operated kidney.
A recent multi-institutional study provided 5-year renal functional outcomes in open partial nephrectomy (OPN) and LPN. Mean WIT was 30.7 and 20 minutes in the LPN and OPN groups, respectively. CKD, defined as preoperative serum creatinine greater than 2.0 mg/dl, was present at a rate of 1.6% and 6.4% in the LPN and OPN groups, respectively. Postoperatively, the incidence of acute renal injury was 0.9% in each group. These data support that 30 minutes of WIT were safe for patients with normal preoperative renal function [2]. Nonetheless, in patients with a contralateral normal functioning kidney, which might play a buffer role for the functional damage generated by the prolonged ischemia, the function of each kidney should be separately evaluated to adjust for the compensation of the contralateral kidney. A recent study with separate functional evaluation of each kidney demonstrated that prolonged WI over 28 minutes resulted in significant functional loss of the affected kidney at 3 months after surgery [31]. In addition, an international expert panel [32] strictly recommended that WIT should be kept to even <20 minutes and that in difficult cases cold ischemia should be started immediately but should not exceed 35 minutes. Therefore, the upper limit of the ischemic time that minimizes renal functional deterioration remains controversial to date. Moreover, there are still debates in human investigations regarding whether the ischemic damage to the affected kidney would be recoverable or whether the damage would be permanent even if WIT is prolonged. From previous large studies suggesting that PN is associated with an increased risk of short- and long-term renal consequences, it is possible that prolonged WI might result in irreversible renal damage for a solitary human kidney [33,34]. Porpiglia et al assessed kidney damage in 18 patients with a normal contralateral kidney 1 year after LPN with a WIT between 31 and 60 minutes [35]. They suggested that mild recovery of the operated kidney was found 1 year after surgery, even with prolonged WI with 99mTc-MAG3 scan. Thus, it would be worthy to separately investigate the functional changes in the affected kidney from total renal function with longer observation periods in patients with a normal contralateral kidney to answer this question. Surgeons should exert extreme efforts to keep WIT as short as possible. When WIT is expected to exceed 30 minutes, an additional method such as renal hypothermia should be considered.
Technically, the retroperitoneal approach is usually preferred for OPN. For LPN, the retroperitoneal approach was associated with decreased operative time (3.5 vs. 5.4 h, p<0.01), blood loss (192 vs. 403 ml, p<0.01), and discharge home (2.3 vs. 3.6 days, p<0.01), with a similar WIT compared with the transperitoneal approach, respectively [36]. Therefore, except for anterior superior and medial masses, retroperitoneal access offered a superior approach. Despite these results, the transperitoneal approach is favored in many centers for LPN, because of the broad suturing space and ability to access tumors in any location with full renal mobilization. Gill et al reported that ease of clamping and unclamping of the hilum by virtue of Satinsky access through the transperitoneal approach allowed them to implement the early unclamping technique, which dramatically improved WIT from 31.9 minutes to 14.4 minutes in LPN [37].
In small and peripheral tumors, manual compression or application of a Kauffman clamp on the renal parenchyma can be sufficient to reduce bleeding during resection in up to 50% of cases [38,39] of OPN. Renal hilar vessel clamping is carried out in 50% to 99% of patients in OPN [2,39-41]. On the other hand, to resect a tumor in a bloodless field and get better outcomes, routine hilar clamping was recommended in LPN [38,39]. Nowadays, LPN without hilar clamping has been tried in selected cases and has been reported to be feasible [42,43].
The perioperative outcomes of LPN tend to be superior to those of OPN. LPN was associated with shorter operative time, decreased operative blood loss, and shorter hospital stay (p<0.01) with the analysis of 1,800 OPN and LPN cases for single T1 renal tumors. Another multicenter study that analyzed the perioperative data of OPN and LPN performed for 10 years demonstrated that the operative time was longer (221 vs. 184 minutes) but estimated blood loss was less (293 vs. 418 cc) and transfusion incidence was lower in the LPN group (p<0.01). The margin-positive rate was comparable in the two groups [44]. The other matched-pair study of OPN and LPN reported that the operative time was shorter (84 vs.150 minutes) and hospitalization time was shorter (5 vs. 7 days) in the LPN group. The margin positive rate and decline in the percentage of baseline hemoglobin were comparable in both groups [45]. The recently reported margin-positive rate of LPN was 0.6% [37].
LPN was considered to have similar or longer ischemic times and more perioperative complications. In a large multicenter study, LPN was associated with longer ischemia time (p<0.01) and more postoperative complications, particularly urological (p<0.01). However, the chance of intraoperative complications was comparable. Renal functional outcomes were similar 3 months after LPN and OPN with 97.9% and 99.6% of renal units retaining function, respectively [2]. In a matched-pair single-center study that compared 100 OPN and LPN cases, the overall complication rate of the LPN group (19%) was comparable to that of the OPN group (14%), but intraoperative complications were higher in the LPN group (10% vs. 3%) [45]. In a study that compared the renal function of the solitary kidney after OPN and LPN, the risk of complications following LPN was 2.54 times that after OPN with a postoperative need for dialysis of 0.6% vs. 10%, respectively [46]. In the Korean multicenter study, the decline of eGFR at the last available follow-up was similar in the OPN and LPN cohorts, whereas the OPN cohort demonstrated shorter ischemic times [44].
However, with simplifying renorrhaphy such as not using bolster [47], using an initial running hemostatic suture under ischemic conditions, reserving thorough parenchymal ligation and pelvicaliceal repair after hilar unclamping [48], and combined technique [37], the median WIT has decreased to 14.4 minutes in recent studies. As the WIT decreased, the postoperative renal function of LPN improved. In the most recent series, Gill et al divided 800 cases of a single surgeon's experience into 3 eras and compared the perioperative and postoperative outcomes. When comparing era one to three, WIT was shorter (31.9, 31.6, and 14.4 minutes, respectively, p<0.01), and the overall rates of postoperative and urological complications were significantly lower in the most recent era despite increasing tumor complexity. Renal function outcomes were superior in era 3, as reflected by a lesser decrease in the estimated GFR (18%, 20%, and 11%, respectively) [37].
The reported oncological outcome of both OPN and LPN is similarly excellent. In the recent data, 3-year cancer-specific survival for patients with a single cT1N0M0 RCC was similar, 99.3% and 99.2% after LPN and OPN, respectively [2]. A multicenter study also demonstrated that OPN and LPN showed similar 5-year recurrent-free survival [44]. In the long-term data, both surgeries also provided similar long-term overall and cancer-specific survival in patients and similar 7-year metastasis-free survival (97.5% vs. 97.3%) after LPN and OPN, respectively [3].
Although LPN has proved to be a worthy alternative with similar oncological outcomes [3] with lower morbidity and faster postoperative recovery [2], much experience is required to perform the surgery for adequate WIT and to get those results. A new alternative is RPN. Since the Da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) was introduced, it has become widespread because of its shorter learning curve than the laparoscopic approach. Now, it is well established for performing radical prostatectomy. As experience is accumulated, the indication of robotic surgery is expanding to PN [49]. Generally, the indications for RPN are the same as for OPN and LPN [50]. Recently, the indications of RPN have also expanded to the multiple renal masses of hereditary kidney cancer patients [51] and the nononcologic field for pediatric RPN [52]. Technically, the transperitoneal approach is used in most RPN series and the retroperitoneal approach was recently introduced (Fig. 1). Hybrid laparoscopic-robotic technique was used in the earlier period. Recent series tend to be fully robotic [53]. Cabello et al describe their three-arm robotic technique with sliding-clip renorrhaphy [54]. The bedside assistant places a clip on the suture, which is then slid down by the console surgeon to obtain perfect tension. With this technique, the console surgeon can fully control the renorrhaphy and WIT can be shorter without knot tying [55]. RPN shows at least equal perioperative outcomes to LPN with shorter overall operative times and WIT as surgeons become more experienced. A positive surgical margin is rare (0-2.3%), and there has been only one reported recurrence following RPN [56,57]. Postoperative renal function and oncological outcomes of RPN are comparable with the LPN series [58]. In recent series, complication rates have ranged from 0% to 17% with rare instances of open conversions. The most common complication is urine leakage [56]. Furthermore, recent reports have suggested that robot assistance confers a particularly strong advantage when attempting to address larger, complex lesions, including those that are predominantly endophytic, centrally located, or directly abutting the hilum (Table 2) [56,59-61]. However, RPN still has limitations. Robotic surgery is more expensive than OPN and LPN in many countries because of the high cost of the Da Vinci robot and because the health care system does not cover it. Another limitation is the requirement for an experienced bedside assistant. Generally, the role of the assistant is important during hilar clamping and releasing with the bulldog clamp or laparoscopic Satinsky. Although efforts to control the hilum by the console surgeon have emerged, such as the use of a fourth arm, it is more difficult than the three-arm approach owing to the crowding of the instruments.
LESS surgery is one of the innovations of laparoscopic surgery. It can minimize patient discomfort and maximize the cosmetic benefit with one small incision. For a kidney tumor, PN can get the advantage because of small specimen differ from RN. The reported WIT of LESS PN was 11 to 29 minutes and that of hybrid robot-assisted LESS PN was 16 to 43 minutes [62,63]. Because most surgeons do not have much experience, keeping the WIT short can be a burden. For this reason, some surgeons perform LESS PN without ischemia [64,65]. Mean operative time was comparable to RPN [50,62-66]. The most common complication was bleeding requiring transfusion [62-66]. Oncological outcomes are promising until cases accumulate. Although the feasibility has been proved, LESS PN is indicated only for small, exophytic, and easily approachable tumors. Wider experience and longer follow-up are necessary to establish the role of this technique.
Radiofrequency ablation (RFA) induces thermal damage by converting radiofrequency waves to heat. The goal of RFA is to induce a temperature of 50-100℃ throughout the tumor [67]. An open, laparoscopic, or percutaneous approach is possible depending on the tumor location. Over a mean follow-up period of 25 months for small renal masses (mean size: 2.4 cm), the overall recurrence-free survival rate was 96.8%, the cancer-specific survival rate was 98.5%, and the overall survival rate was 92.3% [68].
Cryoablation causes tumor destruction by a rapid freeze and thaw cycle at a temperature below minus 20℃ [69]. For the anterior or medial aspect of the kidneys, the laparoscopic cryoablation (LCA) technique is better because these lesions are close to the bowel and other solid organs. In contrast, the percutaneous cryoablation (PCA) technique is preferred for tumors located on the posterior and lateral aspect due to ease of access through the skin [70]. The size and location are predictors of successful treatment. The overall survival rate of PCA was reported to be 92.6% with a cancer-specific survival rate of 100% at a mean follow-up of 22 months [71]. In 3-year follow-up data of LCA, 3-year cancer-specific survival was 98% [72]. The most common complications are pain and paresthesias at the operative site. Limitations are the lack of histologic confirmation of complete tumor ablation and the need for rigorous radiologic follow-up [69].
High-intensity focused ultrasound (HIFU) generates high-intensity ultrasound waves that increase renal tissue temperatures to more than 65℃, leading to tissue necrosis. At the molecular level, tissue damage is mediated by the two mechanisms of thermal and acoustic cavitation [73]. In early results that were confirmed by nephrectomy specimens after HIFU, 15% to 35% of tissue damage was found in the targeted tissue, which indicated incomplete ablations [74]. Additionally, the tissue damage (interstitial hemorrhage, coagulation necrosis, shrinkage of collagen fibers) did not correlate with the administered energy [75]. The reported side effects were grade 3 skin burns and thermal lesion of the small intestine due to poor focusing. Refinement of the technology is required.
Currently, PN is considered a standard treatment for T1a tumors with the benefit of preventing CKD; improving overall survival, especially for the patients younger than 65 years of age; and decreasing the overall mortality rate. For T1b tumors, more clinical data are required to establish the oncological and functional benefits of PN. LPN has come to represent comparable perioperative and oncological outcomes in the recent era. The role of RPN is expected to expand to even complicated or challenging cases. The role of LESS PN is not yet established. Other ablative techniques can be indicated for patients who cannot tolerate the morbidity of conventional surgery and have a high risk of RCC recurrence, such as patients with von Hippel-Lindau syndrome.
Figures and Tables
FIG. 1
Procedures of robotic partial nephrectomy. (A) Marking renal arteries with vessel loops. (B) Confirming the endophytic tumor with intraoperative ultrasound. (C) Clamping renal arteries with bulldog clamps. (D) Resection of tumor. (E, F) Sliding-clip renorrhaphy.
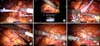
TABLE 2
Outcomes of laparoscopic partial nephrectomy and robotic partial nephrectomy

LPN: laparoscopic partial nephrectomy, RPN: robotic partial nephrectomy, OR time: operative time, EBL: estimated blood loss, T: transperitoneal, R: retroperitoneal, Y: hilar clamping, N: no hilar clamping, CSS: cancer-specific survival, OS: overall survival, RFS: recurrence-free survival, NA: not available, a: p<0.05
References
1. Adami HO, Hunter DJ, Trichopoulos D. Textbook of cancer epidemiology. 2002. New York: Oxford University Press.
2. Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007. 178:41–46.
3. Lane BR, Gill IS. 7-year oncological outcomes after laparoscopic and open partial nephrectomy. J Urol. 2010. 183:473–479.
4. Robson CJ, Churchill BM, Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969. 101:297–301.
5. Touijer K, Jacqmin D, Kavoussi LR, Montorsi F, Patard JJ, Rogers CG, et al. The expanding role of partial nephrectomy: a critical analysis of indications, results, and complications. Eur Urol. 2010. 57:214–222.
6. Levine DZ, Iacovitti M, Robertson SJ, Mokhtar GA. Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2006. 290:R975–R981.
7. Simons JL, Provoost AP, De Keijzer MH, Anderson S, Rennke HG, Brenner BM. Pathogenesis of glomerular injury in the fawn-hooded rat: effect of unilateral nephrectomy. J Am Soc Nephrol. 1993. 4:1362–1370.
8. Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009. 181:55–61.
9. Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008. 112:511–520.
10. Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008. 179:468–471.
11. Coleman JA. Familial and hereditary renal cancer syndromes. Urol Clin North Am. 2008. 35:563–572.
12. Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007. 166:932–940.
13. Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006. 7:735–740.
14. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002. 39:2 Suppl 1. S1–S266.
15. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004. 351:1296–1305.
16. Zini L, Perrotte P, Capitanio U, Jeldres C, Shariat SF, Antebi E, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer. 2009. 115:1465–1471.
17. McKiernan J, Yossepowitch O, Kattan MW, Simmons R, Motzer RJ, Reuter VE, et al. Partial nephrectomy for renal cortical tumors: pathologic findings and impact on outcome. Urology. 2002. 60:1003–1009.
18. Fujii Y, Komai Y, Saito K, Iimura Y, Yonese J, Kawakami S, et al. Incidence of benign pathologic lesions at partial nephrectomy for presumed RCC renal masses: Japanese dual-center experience with 176 consecutive patients. Urology. 2008. 72:598–602.
19. Van Poppel H. Efficacy and safety of nephron-sparing surgery. Int J Urol. 2010. 17:314–326.
20. Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, et al. Renal cell carcinoma guideline. Eur Urol. 2007. 51:1502–1510.
21. Kim JM, Song PH, Kim HT, Park TC. Comparison of partial and radical nephrectomy for pT1b renal cell carcinoma. Korean J Urol. 2010. 51:596–600.
22. Weight CJ, Larson BT, Gao T, Campbell SC, Lane BR, Kaouk JH, et al. Elective partial nephrectomy in patients with clinical T1b renal tumors is associated with improved overall survival. Urology. 2010. 76:631–637.
23. Simmons MN, Weight CJ, Gill IS. Laparoscopic radical versus partial nephrectomy for tumors >4 cm: intermediate-term oncologic and functional outcomes. Urology. 2009. 73:1077–1082.
24. Shikanov S, Lifshitz DA, Deklaj T, Katz MH, Shalhav AL. Laparoscopic partial nephrectomy for technically challenging tumours. BJU Int. 2010. 106:91–94.
25. Novick AC. Renal hypothermia: in vivo and ex vivo. Urol Clin North Am. 1983. 10:637–644.
26. Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001. 166:6–18.
27. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Campbell-Walsh urology. 2007. Philadelphia: Saunders;1686–1753.
28. Bhayani SB, Rha KH, Pinto PA, Ong AM, Allaf ME, Trock BJ, et al. Laparoscopic partial nephrectomy: effect of warm ischemia on serum creatinine. J Urol. 2004. 172:1264–1266.
29. Gill IS, Desai MM, Kaouk JH, Meraney AM, Murphy DP, Sung GT, et al. Laparoscopic partial nephrectomy for renal tumor: duplicating open surgical techniques. J Urol. 2002. 167:469–467.
30. Lane BR, Babineau DC, Poggio ED, Weight CJ, Larson BT, Gill IS, et al. Factors predicting renal functional outcome after partial nephrectomy. J Urol. 2008. 180:2363–2368.
31. Choi JD, Park JW, Choi JY, Kim HS, Jeong BC, Jeon SS, et al. Renal damage caused by warm ischaemia during laparoscopic and robot-assisted partial nephrectomy: an assessment using Tc 99m-DTPA glomerular filtration rate. Eur Urol. 2010. 58:900–905.
32. Becker F, Van Poppel H, Hakenberg OW, Stief C, Gill I, Guazzoni G, et al. Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol. 2009. 56:625–634.
33. Thompson RH, Frank I, Lohse CM, Saad IR, Fergany A, Zincke H, et al. The impact of ischemia time during open nephron sparing surgery on solitary kidneys: a multi-institutional study. J Urol. 2007. 177:471–476.
34. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010. 58:340–345.
35. Porpiglia F, Renard J, Billia M, Musso F, Volpe A, Burruni R, et al. Is renal warm ischemia over 30 minutes during laparoscopic partial nephrectomy possible? One-year results of a prospective study. Eur Urol. 2007. 52:1170–1178.
36. Wright JL, Porter JR. Laparoscopic partial nephrectomy: comparison of transperitoneal and retroperitoneal approaches. J Urol. 2005. 174:841–845.
37. Gill IS, Kamoi K, Aron M, Desai MM. 800 laparoscopic partial nephrectomies: a single surgeon series. J Urol. 2010. 183:34–41.
38. Antonelli A, Cozzoli A, Nicolai M, Zani D, Zanotelli T, Perucchini L, et al. Nephron-sparing surgery versus radical nephrectomy in the treatment of intracapsular renal cell carcinoma up to 7 cm. Eur Urol. 2008. 53:803–809.
39. Patard JJ, Pantuck AJ, Crepel M, Lam JS, Bellec L, Albouy B, et al. Morbidity and clinical outcome of nephron-sparing surgery in relation to tumour size and indication. Eur Urol. 2007. 52:148–154.
40. Gill IS, Matin SF, Desai MM, Kaouk JH, Steinberg A, Mascha E, et al. Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol. 2003. 170:64–68.
41. Thompson RH, Leibovich BC, Lohse CM, Zincke H, Blute ML. Complications of contemporary open nephron sparing surgery: a single institution experience. J Urol. 2005. 174:855–858.
42. Hong HM, Seo IY, Rim JS. Laparoscopic partial nephrectomy without renal arterial clamping. Korean J Urol. 2009. 50:1208–1212.
43. Gill IS, Eisenberg MS, Aron M, Berger A, Ukimura O, Patil MB, et al. "Zero ischemia" partial nephrectomy: novel laparoscopic and robotic technique. Eur Urol. 2011. 59:128–134.
44. Park H, Byun SS, Kim HH, Lee SB, Kwon TG, Jeon SH, et al. Comparison of laparoscopic and open partial nephrectomies in t1a renal cell carcinoma: a Korean multicenter experience. Korean J Urol. 2010. 51:467–471.
45. Marszalek M, Meixl H, Polajnar M, Rauchenwald M, Jeschke K, Madersbacher S. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol. 2009. 55:1171–1178.
46. Lane BR, Novick AC, Babineau D, Fergany AF, Kaouk JH, Gill IS. Comparison of laparoscopic and open partial nephrectomy for tumor in a solitary kidney. J Urol. 2008. 179:847–851.
47. Weight CJ, Lane BR, Gill IS. Laparoscopic partial nephrectomy for selected central tumours: omitting the bolster. BJU Int. 2007. 100:375–378.
48. Nguyen MM, Gill IS. Halving ischemia time during laparoscopic partial nephrectomy. J Urol. 2008. 179:627–632.
49. Guru KA, Hussain A, Chandrasekhar R, Piacente P, Hussain A, Chandrasekhar R, et al. Current status of robot-assisted surgery in urology: a multi-national survey of 297 urologic surgeons. Can J Urol. 2009. 16:4736–4741.
50. Van Haute W, Gavazzi A, Dasgupta P. Current status of robotic partial nephrectomy. Curr Opin Urol. 2010. 20:371–374.
51. Boris R, Proano M, Linehan WM, Pinto PA, Bratslavsky G. Initial experience with robot assisted partial nephrectomy for multiple renal masses. J Urol. 2009. 182:1280–1286.
52. Lee RS, Sethi AS, Passerotti CC, Retik AB, Borer JG, Nguyen HT, et al. Robot assisted laparoscopic partial nephrectomy: a viable and safe option in children. J Urol. 2009. 181:823–828.
53. Kaul S, Laungani R, Sarle R, Stricker H, Peabody J, Littleton R, et al. da Vinci-assisted robotic partial nephrectomy: technique and results at a mean of 15 months of follow-up. Eur Urol. 2007. 51:186–191.
54. Cabello JM, Benway BM, Bhayani SB. Robotic-assisted partial nephrectomy: surgical technique using a 3-arm approach and sliding-clip renorrhaphy. Int Braz J Urol. 2009. 35:199–203.
55. Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol. 2009. 55:592–599.
56. Benway BM, Bhayani SB. Robot-assisted partial nephrectomy: evolution and recent advances. Curr Opin Urol. 2010. 20:119–124.
57. Dulabon LM, Kaouk JH, Haber GP, Berkman DS, Rogers CG, Petros F, et al. Multi-institutional analysis of robotic partial nephrectomy for hilar versus nonhilar lesions in 446 consecutive cases. Eur Urol. 2011. 59:325–330.
58. Shapiro E, Benway BM, Wang AJ, Bhayani SB. The role of nephron-sparing robotic surgery in the management of renal malignancy. Curr Opin Urol. 2009. 19:76–80.
59. Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009. 182:866–872.
60. Rogers CG, Metwalli A, Blatt AM, Bratslavsky G, Menon M, Linehan WM, et al. Robotic partial nephrectomy for renal hilar tumors: a multi-institutional analysis. J Urol. 2008. 180:2353–2356.
61. Rogers CG, Singh A, Blatt AM, Linehan WM, Pinto PA. Robotic partial nephrectomy for complex renal tumors: surgical technique. Eur Urol. 2008. 53:514–521.
62. Aron M, Canes D, Desai MM, Haber GP, Kaouk JH, Gill IS. Transumbilical single-port laparoscopic partial nephrectomy. BJU Int. 2009. 103:516–521.
63. Han WK, Kim DS, Jeon HG, Jeong W, Oh CK, Choi KH, et al. Robot-assisted laparoendoscopic single-site surgery: partial nephrectomy for renal malignancy. Urology. 2011. 77:612–616.
64. Cindolo L, Berardinelli F, Gidaro S, Schips L. Laparoendoscopic single-site partial nephrectomy without ischemia. J Endourol. 2010. 24:1997–2002.
65. Kaouk JH, Goel RK. Single-port laparoscopic and robotic partial nephrectomy. Eur Urol. 2009. 55:1163–1169.
66. White WM, Haber GP, Goel RK, Crouzet S, Stein RJ, Kaouk JH. Single-port urological surgery: single-center experience with the first 100 cases. Urology. 2009. 74:801–804.
67. Berger A, Crouzet S, Canes D, Haber GP, Gill IS. Minimally invasive nephron-sparing surgery. Curr Opin Urol. 2008. 18:462–466.
68. Park S, Anderson JK, Matsumoto ED, Lotan Y, Josephs S, Cadeddu JA. Radiofrequency ablation of renal tumors: intermediate-term results. J Endourol. 2006. 20:569–573.
69. Aron M, Gill IS. Minimally invasive nephron-sparing surgery (MINSS) for renal tumours. Part II: probe ablative therapy. Eur Urol. 2007. 51:348–357.
70. Kimura M, Baba S, Polascik TJ. Minimally invasive surgery using ablative modalities for the localized renal mass. Int J Urol. 2010. 17:215–227.
71. Bandi G, Hedican SP, Nakada SY. Current practice patterns in the use of ablation technology for the management of small renal masses at academic centers in the United States. Urology. 2008. 71:113–117.
72. Gill IS, Remer EM, Hasan WA, Strzempkowski B, Spaliviero M, Steinberg AP, et al. Renal cryoablation: outcome at 3 years. J Urol. 2005. 173:1903–1907.
73. Kohrmann KU, Michel MS, Gaa J, Marlinghaus E, Alken P. High intensity focused ultrasound as noninvasive therapy for multilocal renal cell carcinoma: case study and review of the literature. J Urol. 2002. 167:2397–2403.
74. Marberger M, Schatzl G, Cranston D, Kennedy JE. Extracorporeal ablation of renal tumours with high-intensity focused ultrasound. BJU Int. 2005. 95:Suppl 2. 52–55.
75. Hacker A, Michel MS, Marlinghaus E, Kohrmann KU, Alken P. Extracorporeally induced ablation of renal tissue by high-intensity focused ultrasound. BJU Int. 2006. 97:779–785.
76. Deane LA, Lee HJ, Box GN, Melamud O, Yee DS, Abraham JB, et al. Robotic versus standard laparoscopic partial/wedge nephrectomy: a comparison of intraoperative and perioperative results from a single institution. J Endourol. 2008. 22:947–952.
77. Aron M, Koenig P, Kaouk JH, Nguyen MM, Desai MM, Gill IS. Robotic and laparoscopic partial nephrectomy: a matched-pair comparison from a high-volume centre. BJU Int. 2008. 102:86–92.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download