Abstract
Purpose
To determine the feasibility and safety of robotic partial nephrectomy (RPN), we compared the operative outcomes of patients who had undergone RPN with those of patients who had undergone laparoscopic partial nephrectomy (LPN).
Materials and Methods
Between February 2009 and June 2010, 13 patients underwent transperitoneal RPN (group 1) and 14 patients underwent transperitoneal LPN (group 2) by a single surgeon. The operative outcomes of the 2 groups were compared by using Mann-Whitney U and Fisher's exact tests.
Results
All cases were completed successfully without conversion to open surgery. The mean operative time was 153.2±22.3 and 117.5±32.0 minutes in groups 1 and 2, respectively (p=0.003). The mean robotic console time of group 1 was 101.2±21.5 minutes, and the mean laparoscopic time of group 2 was 86.8±32.3 minutes (p=0.139). The mean warm ischemic time was 35.3±8.5 minutes and 36.4±6.8 minutes in groups 1 and 2, respectively (p=0.823). The mean estimated blood loss was 283.6±113.5 ml and 264.1±163.7 ml (p=0.382), respectively. The mean length of hospital stay was 6.1 and 5.3 days (p=0.290), respectively. The mean tumor size was 2.7±1.2 cm and 2.0±1.2 cm (p=0.035), respectively. The surgical margins were negative in all cases.
Nephron-sparing treatments have been considered as the best therapeutic strategy for localized renal cell carcinoma (RCC), and these procedures have gained popularity [1]. The application of renal ablative treatments, including radiofrequency ablation and cryoablation, has been increasing. These approaches preserve renal function, shorten the duration of hospital stay, and result in early convalescence. However, these treatments have limitations such as the lack of pathologic tumor assessment and uncertain long-term oncologic outcomes [2]. The use of partial nephrectomy has also increased. Although its oncological outcomes are equivalent to those of radical nephrectomy for localized RCC, partial nephrectomy has advantages over radical nephrectomy [3-5]. Patients undergoing radical nephrectomy have a significantly higher chance of developing chronic renal insufficiency than do those undergoing partial nephrectomy. The incidence of renal insufficiency over time is significantly greater in patients undergoing radical nephrectomy.
Laparoscopic partial nephrectomy (LPN) is a less invasive procedure than open surgery. However, it remains a challenging procedure for surgeons without considerable laparoscopic experience. Recently, robotic surgery has found wide applications in the urology field. It allows surgeons to perform difficult operations such as partial nephrectomy. Herein, we present our early experiences of robotic partial nephrectomy (RPN) procedures performed at our institution and compare the operative outcomes of RPN with those of LPN.
Between February 2009 and June 2010, 13 patients underwent transperitoneal RPN (group 1) and 14 patients underwent transperitoneal LPN (group 2) by a single surgeon. The patients were diagnosed with RCC or angiomyolipoma (AML) indistinguishable from RCC in radiologic studies. The operative methods were chosen after obtaining informed consent and agreement for the charges for the operations from the patients. RPN was performed with the da Vinci S® system (Intuitive Surgical, Sunnyvale, CA, USA). In case of a centrally located tumor, a ureteral stent was inserted before the operation. The patients were placed in a 70-degree lateral position under 14 mmHg of CO2 gas insufflation. Usually, 3 robotic ports and 1 assistant port were used. In cases of a tumor in the upper pole of the right kidney, an additional robotic trocar (the third robotic arm) was placed to retract the liver (Fig. 1). In addition, a 30-degree, 10 mm robotic camera was used. The patient cart with robotic arms approached the patient posteriorly. It was docked at a 15-degree angle toward the head of the patient.
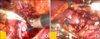
The operative techniques of RPN were similar to those of conventional LPN. A warm ischemia was achieved by placing laparoscopic bulldog clamps at the renal artery. The renal vein was not clamped. Before tumor excision, 12.5 g of mannitol was administered intravenously. The renal tumor was excised approximately 0.5 cm away from the normal tissue. After removal of the tumor, a frozen biopsy of the tumor bed was performed. The bleeding sites were controlled by electric coagulation. The damaged renal pelvocaliceal system was repaired with absorbable Vicryl® 3-0 suture. The renal parenchyma with capsule was sutured with Vicryl® 2-0 attached to Hem-o-lok® clips (Weck closure systems, Research Triangle Park, NC, USA). The continuous suture was initiated from outside the renal parenchyma and continued to compress the renal parenchyma. Hem-o-lok clips were placed after each throw of the running suture. Finally, absorbable clips (Lapra-Ty®, Ethicon Inc., Somerville, NJ, USA) were applied instead of tying knots (Fig. 2). After confirmation of hemostasis, a fibrin sealant (Tisseel®, Baxter AG, Vienna, Austria) and a cellulose mesh (Surgicel®, Ethicon Inc., Somerville, NJ, USA) were placed on the suture sites. The bulldog clamps were removed. After confirmation of hemostasis, fascial suture with Vicryl® 3-0 was performed. A Jackson-Pratt drain was inserted in the operative field. The excised specimen in the laparoscopic pouch was removed with a trocar.
The patients' records were reviewed retrospectively. To compare the operative results of the 2 groups, the Mann-Whitney U test and Fisher's exact test were used. The analysis was performed by using the SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA), with p-values<0.05 considered to indicate statistical significance.
The characteristics of the patients who underwent RPN (group 1) or LPN (group 2) are presented in Table 1. We found no significant differences between the 2 groups. Four cases (30.8%) in group 1 had a history of abdominal operations, including inguinal herniorrhaphy, laparoscopic hemicolectomy, appendectomy, and laparoscopic renal cyst excision. Five cases (35.7%) in group 2 had a history of abdominal operations, including transvaginal hysterectomy, transabdominal hysterectomy, laparoscopic partial nephrectomy, and laparoscopic cholecystectomy.
All cases were completed successfully, without conversion to laparoscopic or open surgery. The mean operative time was 153.2±22.3 minutes and 117.5±32.0 minutes in groups 1 and 2, respectively (p=0.003). The mean robotic console time of group 1 was 101.2±21.5 minutes, and the mean laparoscopic time of group 2 was 86.8±32.3 minutes (p=0.139). The definition of the laparoscopic time was from insertion of the laparoscopic instruments to removal of the instruments. Although the operative time of RPN was longer than that of laparoscopy, the differences in robotic console time and laparoscopic time were not significant. The mean warm ischemic time was 35.3±8.5 minutes and 36.4±6.8 minutes in 9 patients of group 1 and 10 patients of group 2, respectively (p=0.823). The mean estimated blood loss in groups 1 and 2 was 283.6±113.5 ml and 264.1±163.7 ml, respectively (p=0.382). Transfusion was performed in 2 patients (15.4%) in group 1 (p=0.222). There were no major complications in either group. Postoperative pain control was achieved by patient-control analgesia with morphine (40 mg) and ketorolac (150 mg) for 2 days. Additionally, pethidine HCl was administered at mean dosages of 3.3±8.8 mg and 3.5±12.0 in groups 1 and 2, respectively (p=0.299). The mean times to postoperative initiation of ambulation were 1.2±0.4 days and 1.2±0.4 days (p=0.920), and the times to initiation of oral intake were 1.2±0.4 days and 1.1±0.3 days (p=0.504) in groups 1 and 2, respectively. The mean length of hospital stay in groups 1 and 2 was 6.2±1.8 days and 5.3±0.6 days, respectively (p=0.290) (Table 2).
The mean tumor size was 2.7±1.2 cm and 2.0±1.2 cm in groups 1 and 2 (p=0.035). Pathologic results showed 11 cases of RCC and 2 cases of AML in group 1, and 9 cases of RCC, cases of AML, and 1 case of oncocytoma in group 2 (p=0.785). The surgical margins were negative in all cases (Table 3).
Open partial nephrectomy (OPN) is one of the standard modalities of nephron-sparing surgery. Long-term follow-up results of OPN for localized RCC have shown acceptable cancer control effects [6-8]. However, the postoperative recovery period of OPN is similar to that of open radical nephrectomy because of the morbidity due to the large flank incision. The number of LPNs has steadily increased since its introduction by Winfield et al in 1993 [9]. The techniques of LPN duplicate those of OPN. The indications are expanded to more challenging cases, however, including central and hilar tumors, endophytic tumors, tumors greater than 4 cm, tumors in solitary kidneys, and tumors in patients with compromised renal function [10,11]. Concerning oncological outcomes, the 5-year cancer-specific survival rate for T1 RCC was more than 90% in a multicenter study [8,12,13].
LPN has surgical and functional outcomes similar to those of OPN in addition to comparable oncological outcomes. Gill et al presented a comparative study of LPN and OPN, in which LPN had shorter operating time, less estimated blood loss, shorter hospital stay, and longer warm ischemic time [12]. Marszalek et al demonstrated that LPN had shorter warm ischemic time, hospital stay, and operating time in 200 patients undergoing either LPN or OPN [14]. These reports show that LPN is technically feasible. However, LPN still has a steep learning curve to overcome the technical challenges of complete tumor excision with hemostasis and renal reconstruction in a limited renal ischemic time.
Robot-assisted laparoscopic surgery has been widely adapted. It can offer a magnified, three-dimensional view and fully-articulating wristed instruments and enables surgeons to perform difficult operations. RPN was introduced by Gettman et al in 2004 [15]. Among the initial reports, Aron et al presented no proven advantages of RPN over LPN in 12 matched-pair patients [16]. However, due to advances in the surgical technique, favorable operative results and intermediate-term outcomes have been demonstrated in recent reports. The operating time ranged from 83 to 197 minutes. The warm ischemic time ranged from 18 to 28 minutes. The estimated blood loss ranged from 133 to 198 ml. The duration of the hospital stay ranged from 1.9 to 5.2 days. The complication rate ranged from 0% to 17%. The positive surgical margin rate ranged from 0% to 2.3% [17-23]. RPN has advantages in the treatment of complex tumors such as endophytic, hilar, and multiple tumors. Rogers et al reported the feasibility of RPN for hilar tumors in 11 patients in a multi-institutional analysis [24]. Even though the feasibility and safety of RPN has been proven, it does have drawbacks, including the lack of haptic feedback, dependency on an assistant, and high operating costs. Long-term functional and oncological outcomes are needed to make RPN a standard treatment for small renal tumors.
Our initial experience with RPN showed acceptable operative outcomes comparable with those of previous reports [15-23]. However, the mean hospital stay was prolonged. One of the reasons was that the patients required a longer stay under the Korean health care system. We also presented the operative outcomes of RPN and LPN in this comparative study. Although the operative time of RPN was longer than that of LPN, there was no significant difference in robotic console time and laparoscopic time. It might take some time to connect the robotic surgical system to the patient in the RPN group. Unfortunately, the warm ischemic time was slightly long. The reasons for the long ischemic time might be the insertion time of suture materials and inexperienced assistants. We did frozen biopsy in most cases. If we had found a remnant cancer, we would have performed a radical nephrectomy. Two patients in the RPN group had blood transfusions. They had intraperitoneal adhesions from previous abdominal operations, which led to incomplete arterial dissections and clampings and bleeding.
We did not find advantages in the operative results of RPN, which will require further accumulation of experience and technical developments. For example, our technique for RPN had distinguishing operative features for hemostasis. We used a sliding-clip renorrhaphy for calyceal reconstruction and parenchymal compression. When placing the Hem-o-lok clips, it is necessary to pull the suture taut and then push the clip snug against the renal parenchyma [25]. After completion of the suture and confirmation of hemostasis, a fibrin sealant and cellulose mesh were applied to the suture site. Finally, a third layer suture of the fascial covering was performed. This technique has certain advantages, including effective hemostasis, decreased dependence on assistance, and decreased operation and warm ischemic time [18,26].
We acknowledge the limitations of this study. Our study was a retrospective analysis with a small number of patients. Furthermore, long-term follow-up is needed to identify oncologic and functional outcomes of RPN.
Our experiences showed that RPN was technically feasible, and its operative outcomes were comparable to those of LPN. Although the operative time of RPN was longer than that of LPN, there was no significant difference in operative outcomes, including robotic console time and laparoscopic time. To identify advantages of RPN, we must accumulate more experience and make further technical developments. Further studies with long-term follow-up of RPN are also needed to make RPN a standard treatment for localized RCC.
Figures and Tables
FIG. 1
Port sites of robotic partial nephrectomy for a left tumor (A) and a right upper pole tumor (B) in a 70-degree lateral position.
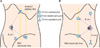
FIG. 2
Operative findings. (A) Reconstructive suture for an opened calyx (arrow). (B) Application of a fibrin sealant (arrow head) after continuous suture using Hem-o-lok clips (arrow).
References
1. Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982-1997). Urology. 2000. 56:58–62.
2. Berger A, Crouzet S, Canes D, Haber GP, Gill IS. Minimally invasive nephron-sparing surgery. Curr Opin Urol. 2008. 18:462–466.
3. Butler BP, Novick AC, Miller DP, Campbell SA, Licht MR. Management of small unilateral renal cell carcinomas: radical versus nephron-sparing surgery. Urology. 1995. 45:34–40.
4. Amling CL. Dilemmas in management of the renal mass. Curr Opin Urol. 2006. 16:315–316.
5. McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002. 59:816–820.
6. Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000. 163:442–445.
7. Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol. 2000. 163:730–736.
8. Permpongkosol S, Bagga HS, Romero FR, Sroka M, Jarrett TW, Kavoussi LR. Laparoscopic versus open partial nephrectomy for the treatment of pathological T1N0M0 renal cell carcinoma: a 5-year survival rate. J Urol. 2006. 176:1984–1988.
9. Winfield HN, Donovan JF, Godet AS, Clayman RV. Laparoscopic partial nephrectomy: initial case report for benign disease. J Endourol. 1993. 7:521–526.
10. Turna B, Aron M, Gill IS. Expanding indications for laparoscopic partial nephrectomy. Urology. 2008. 72:481–487.
11. Park H, Byun SS, Kim HH, Lee SB, Kwon TG, Jeon SH, et al. Comparison of laparoscopic and open partial nephrectomies in T1a renal cell carcinoma: a Korean multicenter experience. Korean J Urol. 2010. 51:467–471.
12. Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007. 178:41–46.
13. Gill IS, Kamoi K, Aron M, Desai MM. 800 laparoscopic partial nephrectomies: a single surgeon series. J Urol. 2010. 183:34–41.
14. Marszalek K, Meixl H, Polajnar M, Rauchenwald M, Jeschke K, Madersbacher S. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol. 2009. 55:1171–1178.
15. Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology. 2004. 64:914–918.
16. Aron M, Koenig P, Kaouk JH, Nguyen MM, Desai MM, Gill IS. Robotic and laparoscopic partial nephrectomy: a matched-pair comparison from a high-volume centre. BJU Int. 2008. 102:86–92.
17. Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009. 182:866–872.
18. Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol. 2009. 55:592–599.
19. Bhayani SB, Das N. Robotic assisted laparoscopic partial nephrectomy for suspected renal cell carcinoma: retrospective review of surgical outcomes of 35 cases. BMC Surg. 2008. 8:16.
20. Ho H, Schwentner C, Neururer R, Steiner H, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: surgical technique and clinical outcomes at 1 year. BJU Int. 2009. 103:663–668.
21. Rogers CG, Menon M, Weise ES, Gettman MT, Frank I, Shephard DL, et al. Robotic partial nephrectomy: a multi-institutional analysis. J Robot Surg. 2008. 2:141–143.
22. Wang AJ, Bhayani SB. Robotic partial nephrectomy versus laparoscopic partial nephrectomy for renal cell carcinoma: single-surgeon analysis of >100 consecutive procedures. Urology. 2009. 73:306–310.
23. Jeong W, Park SY, Lorenzo EI, Oh CK, Han WK, Rha KH. Laparoscopic partial nephrectomy versus robot-assisted laparoscopic partial nephrectomy. J Endourol. 2009. 23:1457–1460.
24. Rogers CG, Metwalli A, Blatt AM, Bratslavsky G, Menon M, Linehan WM, et al. Robotic partial nephrectomy for renal hilar tumors: a multi-institutional analysis. J Urol. 2008. 180:2353–2356.
25. Hong HM, Seo IY, Rim JS. Laparoscopic partial nephrectomy without renal arterial clamping. Korean J Urol. 2009. 50:1208–1212.
26. Benway BM, Bhayani SB. Robot-assisted partial nephrectomy: evolution and recent advances. Curr Opin Urol. 2010. 20:119–124.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download