Abstract
Purpose
The aim of this study was to evaluate whether low-dose anticholinergics combined with an α1-receptor antagonist would continue the effect of an alpha-blocker, decrease the side effects of anticholinergics, and improve the symptoms of lower urinary tract symptoms/benign prostatic hyperplasia (LUTS/BPH).
Materials and Methods
Two hundred nine men with LUTS/BPH with storage symptoms (International Prostate Symptom Score [IPSS] ≥12; storage symptoms ≥4) were randomly assigned in a prospective, multicentered, and single-blind fashion to either the control group (alfuzosin 10 mg, once daily) or the combined group (alfuzosin 10 mg, once daily, and propiverine 10 mg, once daily) for 2 months. IPSS, maximal urinary flow rate (Qmax), and postvoid residual volume (PVR) were used to grade symptoms, side effects, and the impact on quality of life (QoL) at the start of the study and after 1 and 2 months.
Results
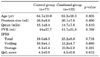
There were no significant differences in patient background, including age, prostate size, Qmax, and PVR, between the control group and the combined group. In the combined group, the IPSS total score and the IPSS storage symptom score were significantly improved compared with the control group. The IPSS voiding symptom score, QoL, Qmax, and PVR did not differ significantly. There were no serious side effects in either group.
Conclusions
Management with an α1-receptor antagonist combined with a low-dose anticholinergic improved the total score and storage symptom score of the IPSS compared with α1-receptor antagonist only group without causing serious side effects. This initial combination medication can be considered an effective and safe treatment modality for LUTS/BPH patients with storage symptoms.
Although the symptoms of lower urinary tract symptoms/benign prostatic hyperplasia (LUTS/BPH) are normally divided into storage symptoms and voiding symptoms, 52-84% of patients have overactive bladder symptoms [1,2]. Moreover, as a patient ages, the natural course increases the frequency of overactive bladder symptoms [3,4]. Traditional management of BPH focuses on voiding symptoms through surgeries like transurethral resection of the prostate (TURP) or the use of medicines such as α1-receptor antagonists or 5-alpha reductase inhibitors. Even though voiding symptoms are alleviated after the use of medicines or TURP, storage symptoms continue for about 30% of patients [5-7]. Theoretically, in this circumstance, administering anticholinergics would help to improve the LUTS/BPH [8,9]. However, adding anticholinergics to patients with LUTS/BPH is not widely applied in clinical practice, because it could aggravate voiding symptoms, increase the risk of acute urinary retention, or increase adverse effects [1]. Also, as elderly patients receive medicines with more anticholinergic ingredients [10], there may be a greater chance of side effects and the severity of the side effects could increase, even though an accurate dosage of anticholinergics can be safe for elderly patients who have a normal urinary flow rate and less residual urine. Even though many methods have been suggested to prevent the side effects of anticholinergics, such as beta-3 agonist [11], purinoreceptor antagonist [12], or COX inhibitor [13], these are still in the development phase, and the clinical trials of these medications are still in debate. The aim of this study was to evaluate the effect and safety of low-dose anticholinergics combined with an α1-receptor antagonist in LUTS/BPH patients with the existence of storage symptoms to decrease the adverse effect of anticholinergics and to improve both the storage symptoms and the voiding symptoms.
This prospective open-label, parallel-group, single-blinded, multicenter study was performed under Institutional Review Board approval of every medical center that participated, and written informed consent was received from all patients.
Inclusion criteria were as follows: presence of LUTS/BPH with International Prostate Symptom Score (IPSS) ≥12 and IPSS storage subscore ≥4. Patients with urinary retention, postvoid residual urine (PVR) ≥200 ml, hydronephrosis, renal impairment, intractable hematuria, prostate cancer, history of pelvic surgery, neurogenic bladder, urethral stricture, or chronic bacterial prostitis were excluded at the start of treatment. The maximal urinary flow rate (Qmax) was not considered as an inclusion criterion. Patients with a medical history of use of a 5ARI within 6 months were also excluded.
Propiverine 10 mg was used as the low-dose anticholinergic. Two hundred fifty men, aged 50 years or older, with LUTS/BPH were enrolled in this study and randomly assigned to either the control group (alfuzosin 10 mg, once daily) or the combined group (alfuzosin 10 mg, once daily, and propiverine 10 mg, once daily) for 2 months in a 2:3 ratio according to consultation with a statistician. The patients were randomized by use of a randomization table.
Before the medication was started, IPSS (total, voiding, and storage scores with QoL), age, blood pressure, comorbidity, combined medications, prostate size (by transrectal ultrasound), Qmax, PVR, and serum prostate-specific antigen (PSA) were evaluated. After the groups had received medication for 1 month, we measured IPSS, Qmax, PVR, and side effects of anticholinergics. The same tests were executed at the end of the study.
The primary endpoint was whether storage symptoms of the IPSS improved after medication for 8 weeks. The secondary endpoint was whether there was a reduction in adverse effects, whether voiding symptoms were affected, and whether there was a decrease in Qmax.
All values are presented as the mean±standard deviation (SD). The significance of differences among groups was determined by using Student's t-test and repeated-measures ANOVA with differences considered significant at p<0.05.
Among the 250 subjects who were randomly assigned, 207 subjects finished the study. The numbers in the control group and combined group were 77 and 132, respectively. There were no significant differences in the patient's characteristics, including age, prostate size, Qmax, and PVR, between the control group and the combined group (Table 1). Although the total, voiding, and storage symptom scores of the IPSS were higher in the combined group than in the control group, the differences were not statistically significant (p>0.05 respectively) (Table 1). The total IPSS score of both groups was significantly improved after 2 months of medication. The total IPSS score of the control group changed from 19.0±6.3 to 11.2±5.6, and that of the combined group changed from 23.2±6.8 to 14.9±4.7. The IPSS storage score of the control group changed from 8.1±3.4 to 4.7±2.3, and that of the combined group changed from 10.0±2.8 to 6.0±2.2. The improvement in the IPSS voiding score and QoL was not significantly different between the two groups at 2 months (Fig. 1). However, compared with the control group, the combined group had significantly greater improvement at 2 months in the IPSS total and storage scores (p=0.031 and p=0.004, respectively) (Fig. 1).
Qmax in uroflowmetry and PVR were improved in both groups at each time point, but were not significantly different compared with the baseline data. The improvement in Qmax and PVR did not differ significantly between the two groups (Fig. 2). During the study there were nine cases of minor adverse events. However, no serious adverse events were reported. The control group had 3 cases (3.9%) and the combined group had 9 cases (6.8%) of side effects (Table 2). Patients who had voiding difficulty complained of postvoid tenesmus (2 cases), straining voiding (2 cases), hesitancy (1 case), and terminal dribbling (1 cases). None of these patients showed specific differences in baseline characteristics, including age, prostate size, Qmax, and PVR. All side effects were mild and there were no cases of stopping medication because of side effects.
In theory, when voiding symptoms are the first priority in LUTS/BPH, an α1-receptor antagonist can be considered as a treatment of choice, and in the case of a large prostate, a 5-alpha reductase inhibitor can be added to reduce the volume of the prostate. When storage symptoms are the first priority in LUTS/BPH, combined therapy with an α1-receptor antagonist and an anticholinergic can be an appropriate choice. These medication therapies could theoretically improve both voiding and storage symptoms [8,9]. In practice, however, clinicians hesitate to administer anticholinergics, especially to patients with severe obstructive symptoms or a large prostate. The use of anticholinergics can make clinicians anxious because of the possibility of worsening existing voiding symptoms, increasing residual urine, or even worse, causing urinary retention. Thus, most clinicians use an alpha-blocker first to ease the urinary symptoms and then use anticholinergics to improve the storage symptoms. As mentioned above, combined therapy from the beginning can lead to an increase in residual urine, which can be a bigger problem. However, even though an increase in residual urine is possible, it is not frequent enough to increase the risk of urinary retention clinically. Moreover, it was reported recently that there is no urinary retention with administration of anticholinergics alone [14-17].
However, earlier published clinical demonstrations have not reflected the real clinical situation, because the testing involved very strict inclusion and exclusion criteria. In other words, the demonstrations excluded all patients with the risk factors that trigger acute urinary retention, such as severe bladder outlet obstruction or a large amount of residual urine. Because there can be huge residual urine, or detrusor underactivity, or myogenic failure as a result of aging in a real-life practice, it is hard to compare the efficacy and safety of anticholinergics [18,19]. In addition, an analysis is needed of the side effects that reduce QoL, such as dry mouth or constipation, and consideration must be given to the changes in pharmacodynamics and pharmacokinetics and polypharmacy of elderly patients.
The incidence of dry mouth, a common side effect of antimuscarinic treatment, was reported by 24% of male bladder outlet obstruction patients treated with tolterodine 4 mg [14]. Another study reported that the incidence was 21% in patients taking a combination of tamsulosin and tolterodine 4 mg [15]. Combination treatment with propiverine 20 mg and doxazosin 4 mg has been reported, and the results showed an incidence of dry mouth of 18.3% [16]. The incidence of dry mouth in our study was only 1.5%, which is quite lower than in other studies. This may have been the result of the low dose of propiverine or the low level of suspicion of the investigator. However, it is clear that a low dose of propiverine causes a low incidence of dry mouth and can increase patience compliance. Also, some evidence suggests that nighttime dosing may reduce the incidence of dry mouth and other adverse events [20].
The most severe side effects of anticholinergics related to urology are acute urinary retention and an increase in residual urine. The most frequent side effect is dry mouth, but constipation, dry eyes, confusion, constipation, somnolence, blurred vision, increased heart rate, angioedema, arrhythmias, disorientation, hallucination, and convulsion can also occur [1]. The incident rates of these side effects increase as the patient ages. Elderly patients taking anticholinergics have a lower metabolism and slower elimination of drugs, increased permeability in the blood-brain barrier, changes in the number and distribution of muscarinic receptors, and age-related deficits compared with younger patients [21]. Also, for nursing home patients, it is reported that 21% to 32% of patients take more than two drugs that have anticholinergic effects, 10% to 17% take more than three, and 5% take more than five [21,22]. Even though these facts address the need for control of the dosage of anticholinergics, few studies have been conducted on the effects of low-dose anticholinergics for patients with LUTS/BPH. Moreover, in therapy for female overactive bladder (OAB), the ratio of stopping a normal dose of anticholinergics because of side effects is quite high. This makes clinicians feel the need for a change in therapy practice [23-26]. We can infer that the ratio is higher in male overactive bladder patients with bladder outlet obstruction. Therefore, the objective of this research was to find methods to treat male overactive bladder that are based on alpha-blocker usage with maintenance of the effect of the alpha-blocker, a decrease in the side effects of anticholinergics, and improvement in the symptoms of LUTS/BPH.
The average dose of propiverine is 20 mg once daily, and it can be used at doses up to 40 mg once daily. Accordingly, we prescribed propiverine 10 mg once daily as a low dose. The improvement in storage symptoms in the control group might have been due to the alpha-blocker. However, low-dose propiverine had only a little of the side effects of anticholinergics and reduced the total score and storage symptom score of the IPSS. The improvement in the voiding symptom score of the IPSS, Qmax, and PVR in the combined group did not differ significantly from the changes in the control group and the side effects remained slight.
This implies that low-dose propiverine is appropriate as a primary therapy for the storage symptoms of LUTS/BPH, especially in elderly patients. In this study, the IPSS score was the main inclusion criterion and Qmax was not considered as an inclusion criterion. Some of the subjects showed a relatively good maximal flow rate. However, it is not uncommon to find LUTS patients who have a good maximal flow rate but want to treat their LUTS. Also, the mean prostate size of the included subjects was only 26 g, and the improvements in storage symptoms after medication may increase voiding volume at the time of evaluating Qmax. These factors might have resulted in the marked improvements in Qmax in this study.
This investigation had a limitation in that it was not a double-blinded study using a placebo. Therefore, the combined group might have felt that they achieved more satisfactory results because of the additional drug, propiverine, compared with the control group. Also, the follow-up period was only 8 weeks, which is relatively shorter than in usual studies. In future research, as Kaplan et al suggested, well-designed, large, double-blind, placebo-controlled, long-term randomized controlled trials are needed to assess the safety and efficacy of low-dose anticholinergics in LUTS/BPH [15].
According to this randomized study, alfuzosin with low-dose propiverine was superior to alfuzosin alone for IPSS total and storage scores in LUTS/BPH patients with storage symptoms. The combination of an α1-receptor antagonist with a low-dose anticholinergic can be considered an effective and safe treatment modality from the start that does not result in more serious side effects in LUTS/BPH patients with storage symptoms.
Figures and Tables
FIG. 1
Change from baseline to months 1 and 2 in IPSS total, voiding, and storage scores and quality of life index in the control and combined groups. IPSS: International Prostate Symptom Score, a: p<0.05.

FIG. 2
Change in maximal flow rate (upper) and post-voided residual urine volume (lower) in the control and combined groups at each time point. At the 0.05 level, the difference in the population means was not significantly different than the test difference. Data are mean values and were analyzed by repeated-measures ANOVA.

References
1. Chapple CR, Smith D. The pathophysiological changes in the bladder obstructed by benign prostatic hyperplasia. Br J Urol. 1994. 73:117–123.
2. Park HK, Park H, Cho SY, Bae J, Jeong SJ, Hong SK, et al. The prevalence of benign prostatic hyperplasia in elderly men in Korea: a community-based study. Korean J Urol. 2009. 50:843–847.
3. Milsom I, Abrams P, Cardozo L, Roberts RG, Thüroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001. 87:760–766.
4. Kim HW, Seo SI, Ko JS, Jung JH, Lee JY. Incidence of overactive bladder in benign prostatic hyperplasia and the efficacy of combination therapy of alpha blocker with tolterodine. Korean J Urol. 2003. 44:1006–1010.
5. Abrams PH, Farrar DJ, Turner-Warwick RT, Whiteside CG, Feneley RC. The results of prostatectomy: a symptomatic and urodynamic analysis of 152 patients. J Urol. 1979. 121:640–642.
6. Kakizaki H, Machino R, Koyanagi T. Clinical experience in lower urinary tract symptoms. BJU Int. 2001. 88:Suppl 2. 23–26.
7. Seaman EK, Jacobs BZ, Blaivas JG, Kaplan SA. Persistence or recurrence of symptoms after transurethral resection of the prostate: a urodynamic assessment. J Urol. 1994. 152:935–937.
8. Engström G, Henningsohn L, Walker-Engström ML, Leppert J. Impact on quality of life of different lower urinary tract symptoms in men measured by means of the SF 36 questionnaire. Scand J Urol Nephrol. 2006. 40:485–494.
9. Sountoulides P, van Dijk MM, Wijkstra H, de la Rosette JJ, Michel MC. Role of voiding and storage symptoms for the quality of life before and after treatment in men with voiding dysfunction. World J Urol. 2010. 28:3–8.
10. Chapple CR, Rechberger T, Al-Shukri S, Meffan P, Everaert K, Huang M, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004. 93:303–310.
11. Otsuka A, Shinbo H, Hasebe K, Matsumoto R, Ozono S. Effects of a novel beta(3)-adrenoceptor agonist, AJ-9677, on relaxation of the detrusor muscle: an in vitro study. Int J Urol. 2008. 15:1072–1076.
12. Zagorodnyuk VP, Brookes SJ, Spencer NJ, Gregory S. Mechanotransduction and chemosensitivity of two major classes of bladder afferents with endings in the vicinity to the urothelium. J Physiol. 2009. 587:3523–3538.
13. Jang J, Park EY, Seo SI, Hwang TK, Kim JC. Effects of intravesical instillation of cyclooxygenase-2 inhibitor on the expression of inducible nitric oxide synthase and nerve growth factor in cyclophosphamide-induced overactive bladder. BJU Int. 2006. 98:435–439.
14. Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol. 2006. 175:999–1004.
15. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006. 296:2319–2328.
16. Lee KS, Choo MS, Kim DY, Kim JC, Kim HJ, Min KS, et al. Combination treatment with propiverine hydrochloride plus doxazosin controlled release gastrointestinal therapeutic system formulation for overactive bladder and coexisting benign prostatic obstruction: a prospective, randomized, controlled multicenter study. J Urol. 2005. 174:1334–1338.
17. Staskin DR, Rosenberg MT, Dahl NV, Polishuk PV, Zinner NR. Effects of oxybutynin transdermal system on health-related quality of life and safety in men with overactive bladder and prostate conditions. Int J Clin Pract. 2008. 62:27–38.
18. Irani J. Anticholinergic drugs in patients with bladder outlet obstruction and lower urinary tract symptoms: Where do we stand in 2006? Eur Urol. 2006. 50:653–654.
19. Novara G, Galfano A, Ficarra V, Artibani W. Anticholinergic drugs in patients with bladder outlet obstruction and lower urinary tract symptoms: a systematic review. Eur Urol. 2006. 50:675–683.
20. Rackley R, Weiss JP, Rovner ES, Wang JT, Guan Z. Nighttime dosing with tolterodine reduces overactive bladder-related nocturnal micturitions in patients with overactive bladder and nocturia. Urology. 2006. 67:731–736.
21. Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging. 1993. 3:335–348.
22. Hong WS, Chung H, Lee JM, Kim TW, Kim HS, Kim HJ, et al. Polypharmacy and central nervous system adverse effects of anticholinergic agents in the men with benign prostate hyperplasia. J Korean Continence Soc. 2007. 11:24–29.
23. Abrams P, Freeman R, Anderström C, Mattiasson A. Tolterodine, a new antimuscarinic agent: as effective but better tolerated than oxybutynin in patients with an overactive bladder. Br J Urol. 1998. 81:801–810.
24. Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A medium-term analysis of the subjective efficacy of treatment for women with detrusor instability and low bladder compliance. Br J Obstet Gynaecol. 1997. 104:988–993.
25. Milsom I, Abrams P, Cardozo L, Roberts RG, Thüroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001. 87:760–766.
26. Versi E, Appell R, Mobley D, Patton W, Saltzstein D. The Ditropan XL Study Group. Dry mouth with conventional and controlled-release oxybutynin in urinary incontinence. Obstet Gynecol. 2000. 95:718–721.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download