Abstract
Purpose
With the use of 12 months of follow-up data, this study was conducted to evaluate the efficacy of photoselective vaporization of the prostate (PVP) with the 120 W Greenlight high performance system (HPS) laser for the treatment of symptomatic benign prostatic hyperplasia.
Materials and Methods
Data were collected from 104 patients who were diagnosed with benign prostatic hyperplasia and who underwent PVP with the 120 W Greenlight HPS Laser. Postoperative parameters, including International Prostate Symptom Score (IPSS), quality of life (QoL) score, maximum urinary flow rate (Qmax), and postvoid residual volume (PVR), were assessed and compared with preoperative baseline values.
Results
The mean age of the patients was 71.1±7.7. The baseline mean prostate-specific antigen level was 3.8±2.7 ng/ml, the mean prostate size was 43.9±20.6 g, the mean preoperative IPSS was 18.4±8.5, the mean QoL score was 4.1±1.0, the mean Qmax was 9.9±5.5 ml/sec, and the mean PVR was 89.6±207.1 ml. During surgery, the mean operation time was 21.8±11.3 minutes, the mean lasing time was 16.9±10.5 minutes, and the mean total applied energy was 170,068±63,181 J. At 1 month, significant improvements were observed in total IPSS (11.5±6.7, p<0.05), voiding symptom score (6.1±5.4, p<0.05), and QoL score (2.2±1.5, p<0.05); however, there were no significant improvements in storage symptom score (4.8±3.8, p=0.06), Qmax (12.6±10.2, p=0.06), and PVR (40.1±30.5, p=0.41). However, 3 months after surgery, all postoperative follow-up parameters showed significant improvements, and the 6- and 12-month data showed sustained improvement of postoperative follow-up parameters.
The prevalence of benign prostatic hyperplasia (BPH) has shown a progressive increase, owing much to an increase in the elderly population, advancement in diagnostic methods, economic growth, and desire for a better quality of life. Ninety percent of men aged 85 or older are believed to have BPH [1], of whom 25% to 30% need therapy [2]. Treatment of lower urinary tract symptoms (LUTS) caused by BPH has advanced over the years, and numerous treatment options are now available. Most BPH can be treated effectively with endoscopic surgery, with the exception of a large prostate volume, exceeding 100 g; until now, transurethral resection of prostate (TURP) has been the standard treatment of choice [3,4]. However, complications such as postoperative bleeding, urethral stricture, urinary incontinence, retrograde ejaculation, and transurethral resection syndrome (TUR syndrome) have been reported at a high rate after TURP [5-7]. For this reason, numerous less-invasive alternative laser therapies have been proposed. However, most methods were not as effective as TURP and complication rates were unacceptably high [8].
The 60 W potassium-titanyl-phosphate (KTP) laser photoselective vaporization prostatectomy (PVP) was introduced in the late 1990s, and the high powered 80 W KTP laser followed in the year 2000. This laser differs from the others in that it has a high absorption affinity for hemoglobin and a low absorption affinity for water. The efficacy and safety of the laser are similar to those of traditional TURP, making it an ideal substitute for vaporization of prostate tissue [3,9-11].
Recently, introduction of the 120 W lithium triborate (LBO) or the Greenlight high performance system (HPS) laser (American Medical Systems, Minnetonka, MN, USA), for which PVP efficacy and safety have been proven by many studies, was greeted with much applause [12,13]. The purpose of this study was to report on the long-term outcome of Greenlight HPS laser PVP in BPH patients on the basis of experience from a single institute.
From March 2009 to November 2010, 104 patients with symptoms consistent with LUTS were treated with Greenlight HPS (American Medical Systems, Minnetonka, MI, USA) laser PVP in our center. Inclusion criteria were symptoms that persisted after appropriate medical therapy, refusal of proper medication due to side effects, obstruction on urodynamic studies, hematuria originating from the prostate, bladder stones, and persistent urinary tract infections. All patients were followed up for more than 12 months after surgery. All patients were assessed with a complete medical history, physical examination, International Prostate Symptom Score (IPSS), maximum urinary flow rate (Qmax), postvoid residual volume (PVR), transrectal ultrasonography (TRUS), prostate-specific antigen (PSA), complete blood cell count including hemoglobin, urine analysis, and urodynamic study. Those with a palpable nodule, with a PSA value greater than 4 ng/ml, with a suspicious hypoechoic lesion in TRUS findings, who underwent concomitant transrectal prostate needle biopsy, and with pathology-proven prostate cancer were omitted from our study.
General or spinal anesthesia was used, and surgery was performed by a single surgeon. A continuous running irrigation system 22 Fr resectoscope with a 30° lens and a 75° laser fiber was used. For continuous irrigation for a better surgical view, 0.9% normal saline was used. The 120 W HPS laser system (GreenLight, Laserscope®) was used, and vaporization was maintained at a distance of 1 mm from the prostate tissue for an optimal vaporization effect. Vaporization was started at the bladder neck in a clockwise manner, pulling the resectoscope further out and rotating the laser fiber simultaneously with power set at 60-120 W. Power was set at 30 W for coagulation. All prostate tissue causing obstruction was removed until a fine surgical cavity was formed, as in TURP. An 18 Fr urethral catheter was placed after the operation and it was removed the next day, taking into consideration the degree of hematuria.
Postoperative Qmax, PVR, and IPSS with QoL score were obtained at 1, 3, 6, and 12 months after surgery. Operation time, applied energy, and duration of catheterization were obtained. Postoperative Qmax, PVR, IPSS, and QoL score were compared with preoperative values by using the Wilcoxon signed-rank test; p-values of less than 0.05 were defined as statistically significant.
A total of 104 patients met the inclusion criteria. The patients' mean age was 71.1 years, and the mean follow-up period was 15.2 months (range, 12-18 months). Preoperative parameters were as follows: IPSS: 18.4±8.5, QoL: 4.1±1.0, Qmax: 9.9±5.5 ml/s, PVR: 89.6±207.1 ml, PSA: 3.8±2.7 ng/ml, and prostate volume: 43.9±20.6 g (Table 1). Mean operation time was 21.8±11.3 minutes, mean lasing time was 16.9±10.5 minutes, and mean applied energy was 170,068±63,181 J. Mean catheterization duration was 1.01±0.2 days (Table 2). One month after surgery, subjective and objective follow-up parameters were assessed and statistical analysis was performed. Compared with the preoperative data, statistically significant improvements were observed in total IPSS (11.5±6.7, p<0.05), the voiding symptom score (6.1±5.4, p<0.05), and the QoL score (2.2±1.5, p<0.05); however, there were no statistically significant improvements in the storage symptom score (4.8±3.8, p=0.06), Qmax (12.6±10.2, p=0.06), and PVR (40.1±30.5, p=0.41). Three months after the operation, all parameters were reassessed, and the statistical analysis was performed again. Compared with the preoperative data, significant improvements were observed in total IPSS (9.4±7.1, p<0.05), the voiding symptom score (5.7±4.5, p<0.05), the storage symptom score (3.9±3.8, p<0.05), the QoL score (1.8±1.2, p<0.05), Qmax (13.3±8.7, p<0.05), and PVR (32.9±34.6, p<0.05). All postoperative follow-up parameters were assessed again at 6 and 12 months after surgery. The 6-month total IPSS was 6.9±1.5, the voiding symptom score was 3.1±2.7, the storage symptom score was 3.8±2.2, the QoL score was 1.7±1.5, Qmax was 14.4±7.3 ml/s, and PVR was 29.3±28.9 ml. The 12-month total IPSS was 5.0±6.1, the voiding symptom score was 2.5±4.4, the storage symptom score was 2.5±2.4, the QoL score was 1.0±0.8, Qmax was 19.9±7.5 ml/s, and PVR was 13.7±1.2 ml. All of the above 3, 6, and 12-month postoperative values were statistically significant when compared with baseline values and were sustained throughout the follow-up period (Fig. 1, 2). The only major postoperative complication in our study was mild dysuria (n=14, 13.4%), which improved with conservative care. There were no complications such as delayed hematuria or obstructive retention with blood clot.
The prevalence of BPH has shown a progressive increase, owing much to an increase in the elderly population, advancement in diagnostic methods, economic growth, and desire for a better quality of life. Ninety percent of men aged 85 or older are believed to have BPH [1], of whom 25% to 30% need therapy [2].
Despite the large number of alternative procedures available, TURP remains the gold standard surgical technique for experienced surgeons. However, with the recent increase of interest in nonsurgical methods and minimally invasive therapy, many laser-using techniques for the treatment of BPH have been developed.
The neodymium:yttrium-aluminum-garnet (Nd:YAG) laser generates a frequency of 1,064 nm and is not selectively absorbed by prostate tissue. The Nd:YAG laser energy is mainly converted into heat and causes a deep coagulation zone. The major disadvantage of the Nd:YAG laser is the requirement for prolonged bladder drainage, longer hospitalization, poor maintenance of initial symptom improvements, and higher rates of reoperation [14].
In 1998, Malek et al introduced the KTP laser. The photoselectivity of the green 532 nm laser wavelength is based on the fact that this visible wavelength is strongly absorbed within the very superficial layer of tissue [15]. The KTP laser emits visible green light at a wavelength of 532 nm, which is strongly absorbed by oxyhemoglobin, but hardly at all by water, which prevents the beam from penetrating into deeper tissue layers. The heat generated by absorption of the KTP laser energy leads to the formation of vapor bubbles inside the targeted tissue. Continued exposure of the targeted area to KTP laser energy leads to progressive vaporization of the newly exposed deeper layers of tissue. Tissue ablation is visible in real time by the continuous release of bubbles from the tissue surface during the laser procedure. Therefore, the KTP laser can immediately remove obstructive prostatic adenoma without bleeding or deep tissue necrosis. Many recent studies have reported that values for IPSS, Qmax, and QoL after the KTP laser are equivalent to those after TURP [8,10,11,16]. In addition, the safety of patients taking anticoagulants has been confirmed [17,18].
On the other hand, the primary disadvantage of the 80 W KTP laser is that the vaporizing power of the tissue is very low, which lengthens the operation time. To overcome this shortcoming, the 120 W HPS laser was introduced in 2006.
When the 80 W KTP laser is used, the operation time for treatment of BPH shows a wide range [16,19]. According to one domestic report, the mean lasing time was 44.9 min; however, our mean lasing time in the present study was 21.8 min [20]. We were able to conclude that use of the 120 W HPS laser resulted in a shortened operation time.
Bouchier-Hayes reported that in a trial of 80 W laser PVP with 120 patients, IPSS showed a decrease from a preoperative value of 25.3 to 8.9 after 12 months and Qmax increased from 8.8 ml/s to 18.6 ml/s [16]. In the present study with 120 W HPS laser PVP, IPSS changed from a preoperative value of 18.4 to 11.5, 9.4, 6.9, and 5.0 at 1, 3, 6, and 12 months after surgery, respectively; and Qmax changed from 9.9 ml/s preoperatively to 12.6, 13.3, 14.4, and 19.9 ml/s after 1, 3, 6, and 12 months, respectively. Compared with the reported results for the use of the 120 W HPS laser by Al-Ansari et al, Son et al and Ko et al, the decrease in IPSS and increase in Qmax were similar in this study [21-23].
Spaliviero et al reported postoperative complications of 70% for nonspecific hematuria, which disappeared within 1 week, and of 14.3% for retrograde ejaculation after 120 W HPS laser PVP [13]. Retrograde ejaculation rates of 70% to 100% after TURP and of 96% after HoLEP have been reported [24]. On the basis of this report, 120 W HPS laser PVP has a low incidence of sexual dysfunction by retrograde ejaculation; therefore, we can recommend that this procedure can be performed safely in patients who wish to maintain their sexual ability.
In general, in cases of preoperative urethral catheterization due to urinary retention, intake of anticoagulant medication and a large prostate show low satisfaction with increased complication rates after the operation. One ex-vivo report on the 120 W HPS laser showed a higher hemorrhagic rate compared with the 80 W HPS laser [25]. However, in research recently reported by the international Greenlight laser user group, in cases in which the size of the prostate was over 80 g, IPSS decreased from a preoperative value of 22.1 to a postoperative value of 8, Qmax increased from 5.8 to 19.7, and PVR decreased from 118.4 to 55.5. In that study, data were collected from 65 urinary retention patients, 70 patients on anticoagulants, and 52 patients with BPH over 80 g, and IPSS, Qmax, and PVR were noted pre- and postoperatively. Other satisfactory results have also been reported [26].
The results of these reports suggest that the 120 W HPS laser can be applied safely to patients with a history of urinary retention, to patients taking anticoagulant medication, and in patients with a large prostate over 80 g.
In our study, IPSS decreased from a preoperative value of 24.0 to a postoperative value of 6.2, Qmax increased from 6.69 to 10.64, and PVR decreased from 102.5 to 35.7 in 11 patients with a prostate size over 80 g.
The interesting result of our study was that some postoperative follow-up parameters showed no significant improvement at 1 month after the operation; however, 3 months later, all parameters showed significant improvement. We think that this result was due to postoperative tissue edema or bladder irritative symptoms remaining up to 1 month after the operation. Therefore, we recommend observation of symptomatic improvements for at least 1 month after surgery.
Among the participants of our study, two patients underwent the operation because their satisfaction with urination was low and they strongly wanted the operation, although we did not advise the operation because their Qmax was high. After the operation, however, there was a significant improvement in IPSS and QoL and patient satisfaction was high for all of them.
Interest in nonsurgical methods and minimally invasive therapy has shown a recent increase, and many techniques using lasers for the treatment of BPH have been developed. We conducted an analysis of the long-term postoperative effects of 120 W HPS laser PVP procedures and confirmed improved, or at least the same, results compared with TURP procedures or the conventional KTP laser. Our experiences suggest that the 120 W HPS laser PVP procedure is safe and effective. A longer period of data observation of a larger population and postoperative complications should be evaluated next.
Figures and Tables
FIG. 1
Changes in preoperative and postoperative IPSS scores. IPSS: International Prostate Symptom Score, Preop: preoperative characteristics, PVP: photoselective vaporization of prostate, QoL: quality of life, a: p<0.05 compared with preoperative IPSS scores.
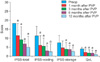
FIG. 2
(A) Preoperative and postoperative values of Qmax. (B) Preoperative and postoperative PVR. Qmax: maximum urinary flow
rate, PVR: postvoid residual volume, Preop: preoperative, Postop: postoperative.

References
1. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984. 132:474–479.
2. Levy A, Samraj GP. Benign prostatic hyperplasia: when to "watch and wait," when and how to treat. Cleve Clin J Med. 2007. 74:Suppl 3. S15–S20.
3. de la Rosette JJ, Alivizatos G, Madersbacher S, Perachino M, Thomas D, Desgrandchamps F, et al. EAU Guidelines on benign prostatic hyperplasia (BPH). Eur Urol. 2001. 40:256–263.
4. Roehrborn CG, Bartsch G, Kirby R, Andriole G, Boyle P, de la Rosette J, et al. Guidelines for the diagnosis and treatment of benign prostatic hyperplasia: a comparative, international overview. Urology. 2001. 58:642–650.
5. Borboroglu PG, Kane CJ, Ward JF, Roberts JL, Sands JP. Immediate and postoperative complications of transurethral prostatectomy in the 1990s. J Urol. 1999. 162:1307–1310.
6. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur Urol. 2006. 50:969–979.
7. Reich O, Gratzke C, Stief CG. Techniques and long-term results of surgical procedures for BPH. Eur Urol. 2006. 49:970–978.
8. Te AE. The development of laser prostatectomy. BJU Int. 2004. 93:262–265.
9. Bachmann A, Ruszat R, Wyler S, Reich O, Seifert HH, Müller A, et al. Photoselective vaporization of the prostate: the basel experience after 108 procedures. Eur Urol. 2005. 47:798–804.
10. Malek RS, Kuntzman RS, Barrett DM. Photoselective potassium-titanyl-phosphate laser vaporization of the benign obstructive prostate: observations on long-term outcomes. J Urol. 2005. 174:1344–1348.
11. Te AE, Malloy TR, Stein BS, Ulchaker JC, Nseyo UO, Hai MA, et al. Photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia: 12-month results from the first United States multicenter prospective trial. J Urol. 2004. 172:1404–1408.
12. Malek RS, Kuntzman RS, Barrett DM. High power potassium-titanyl-phosphate laser vaporization prostatectomy. J Urol. 2000. 163:1730–1733.
13. Spaliviero M, Araki M, Wong C. Short-term outcomes of Greenlight HPS laser photoselective vaporization prostatectomy (PVP) for benign prostatic hyperplasia (BPH). J Endourol. 2008. 22:2341–2347.
14. Ruszat R, Seitz M, Wyler SF, Müller G, Rieken M, Bonkat G, et al. Prospective single-centre comparison of 120-W diode-pumped solid-state high-intensity system laser vaporization of the prostate and 200-W high-intensive diode-laser ablation of the prostate for treating benign prostatic hyperplasia. BJU Int. 2009. 104:820–825.
15. Malek RS, Barrett DM, Kuntzman RS. High-power potassium-titanyl-phosphate (KTP/532) laser vaporization prostatectomy: 24 hours later. Urology. 1998. 51:254–256.
16. Bouchier-Hayes DM, Van Appledorn S, Bugeja P, Crowe H, Challacombe B, Costello AJ. A randomized trial of photoselective vaporization of the prostate using the 80-W potassium-titanyl-phosphate laser vs transurethral prostatectomy, with a 1-year follow-up. BJU Int. 2010. 105:964–969.
17. Ruszat R, Wyler S, Forster T, Reich O, Stief CG, Gasser TC, et al. Safety and effectiveness of photoselective vaporization of the prostate (PVP) in patients on ongoing oral anticoagulation. Eur Urol. 2007. 51:1031–1038.
18. Sandhu JS, Ng CK, Gonzalez RR, Kaplan SA, Te AE. Photoselective laser vaporization prostatectomy in men receiving anticoagulants. J Endourol. 2005. 19:1196–1198.
19. Ruszat R, Seitz M, Wyler SF, Abe C, Rieken M, Reich O, et al. GreenLight laser vaporization of the prostate: single-center experience and long-term results after 500 procedures. Eur Urol. 2008. 54:893–901.
20. Choo SH, Han DH, Lee SW. The efficacy and safety of KTP photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia: the 2-year results. Korean J Urol. 2008. 49:831–836.
21. Al-Ansari A, Younes N, Sampige VP, Al-Rumaihi K, Ghafouri A, Gul T, et al. GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia: a randomized clinical trial with midterm follow-up. Eur Urol. 2010. 58:349–355.
22. Son H, Ro YK, Min SH, Choo MS, Kim JK, Lee CJ. Modified vaporization-resection for photoselective vaporization of the prostate using a GreenLight high-performance system 120-W Laser: the Seoul technique. Urology. 2011. 77:427–432.
23. Ko DW, Jeong BC, Son H. Initial experiences with a new 120 W Greenlight™ high-power system for photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia in Korea. Korean J Urol. 2009. 50:1089–1094.
24. Ahmed HU, Thwaini A, Shergill IS, Hammadeh MY, Arya M, Kaisary AV. Greenlight prostatectomy: a challenge to the gold standard? A review of KTP photoselective vaporization of the prostate. Surg Laparosc Endosc Percutan Tech. 2007. 17:156–163.
25. Heinrich E, Wendt-Nordahl G, Honeck P, Alken P, Knoll T, Michel MS, et al. 120W Lithium triborate laser for photoselective vaporization of the prostate: comparison with 80w potassium-titanyl-phosphate laser in an ex-vivo model. J Endourol. 2010. 24:75–79.
26. Reich O, Bachmann A, Siebels M, Hofstetter A, Stief CG, Sulser T. High power (80 W) potassium-titanyl-phosphate laser vaporization of the prostate in 66 high risk patients. J Urol. 2005. 173:158–160.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download