Abstract
Primary tumors arising from the spermatic cord are very rare. Mesothelioma derives from the mesothelial cells lining the serous membrane, such as the pleura, peritoneum, and tunica vaginalis of testis. Paratesticular malignant mesothelioma (MM), which usually presents as a hydrocele or intrascrotal mass, accounts for 0.3% to 1.4% of MMs. MMs of the spermatic cord account for less than 10% of paratesticular MMs. We report a case of MM of the spermatic cord in a 65-year-old man who primarily presented to the hospital with a left inguinal mass. Following the diagnosis after surgery, he was found to have a contralateral right inguinal mass and died in 6 months. Despite their rare occurrence in the spermatic cord, MMs need to be suspected, especially in patients with a history of asbestos exposure.
Malignant mesothelioma (MM) derives from the mesothelial cells lining serous membranes, such as the pleura, peritoneum, and tunica vaginalis of testis. Paratesticular MM, which usually presents as a hydrocele or intrascrotal mass, accounts for 0.3% to 1.4% of MMs [1]. Around 35% to 40% of patients have a history of direct occupational or familial asbestos exposure [2]. This tumor rarely presents as a spermatic cord mass, and only about 10 cases have been reported in the past [3]. Here we report a case of a patient diagnosed with MM arising from the spermatic cord. The diagnosis was followed by the patient's death in 6 months.
A 65-year-old man presented to our outpatient department with a painless, palpable mass in his left inguinal area that had persisted for 2 months. The mass was insidious in onset and had increased gradually in size. He had no bowel or bladder symptoms and no history of previous surgery or trauma to the region. He did have a history of 4 years of employment at a foundry job 10 years previously; thus, he had been exposed to asbestos for a duration of 4 years.
On the physical examination, a 3×3 cm mass was palpated in his left inguinal area. The mass was hard, globular, smooth, and nontender, and the lower margins were not palpable below the inguinal ligament. A hard spermatic cord was palpated. The scrotum was normal except for a slightly enlarged, nontender left testis. The laboratory examinations showed a normal complete blood count and normal renal and liver functions. Alpha fetoprotein was 3.07 ng/ml (normal <20 ng/ml) and the beta human chorionic gonadotropin level was 1.20 mIU/ml (normal <5 mIU/ml). The results of a chest X-ray and following chest CT were normal. Ultrasound examination of the abdomen and scrotum showed a 3.0×3.3×1.8 cm nodal mass in the left inguinal area. The right testis was 3.0×2.2×4.9 cm in size, whereas the left testis was enlarged to 3.3×2.7×4.9 cm, contained a little hydrocele, and had normal echogenicity and vascularity. Computed tomography (CT) of the abdomen showed a left inguinal mass and bilateral small renal cysts but no abnormalities in other intraabdominal organs (Fig. 1).
The patient underwent left radical orchiectomy. At the peritoneal surface of the internal inguinal ring, three small nodular masses were noted and excised. Grossly, the left orchiectomized specimen was composed of a 5.0×6.0 cm testis attached to a 12 cm length spermatic cord and a detached tunica vaginalis hydrocele sac. At the spermatic cord, there was a nodular mass measuring 5.0×3.2×2.5 cm. The outer surface of the mass was relatively well capsulated and the cut surface had a shiny white color. The testis architecture was well preserved (Fig. 2). The excised nodular tissues at the internal ring were measured 1.5×1.0×0.3 cm in the large one and 0.5×0.2×0.3 cm in the small one. Microscopically, the spermatic cord mass and peritoneal nodules were relatively well demarcated and desmoplastic. The mass was composed of foci of epithelial tumor cells with mostly tubular or papillary patterns and obvious sarcomatous tumor cells. The tumor cells showed high-grade nuclei and atypical mitotic figures (3-4/10 HPF) (Fig. 3). The epithelial and sarcomatoid tumor cells were immunoreactive to cytokeratin, calretinin, vimentin, and mesothelial antibody (HBME-1) but were not reactive to carcinoembryonic antigen (CEA) (Fig. 4). The peritumoral resection margin was partially penetrated by the tumor. The cord resection margin was free of tumor. No lymphovascular invasion was seen. The testicular parenchyma was unremarkable. All of these findings were compatible with MM arising from the left spermatic cord.
We recommended that the patient be managed further with pelvic lymphadenectomy and adjunctive chemotherapy, which he refused. At 3 months after the operation, he returned to our outpatient department with the complaint of abdominal bulging and discomfort. On the physical examination, the abdomen showed marked bulging. The following CT showed a 1.8 cm oval-shaped nodule in his right inguinal area, which suggested MM arising from the contra-lateral spermatic cord. Also, he had peritoneal carcinomatosis with a large amount of ascitic fluid collection, peritoneal thickening, misty mesentery, and a centralization of the small bowel. He was referred to our hemato-oncology department for cisplatin-based intraperitoneal chemotherapy. He underwent one cycle of chemotherapy with some improvement. He died 6 months after the operation.
Paratesticular MMs are distributed over a wide age (range, 7-87 years), but mostly occur in patients in the sixth to eighth decades of life [4]. Most paratesticular MM arises in the tunica vaginalis [4,5], a peritoneal outpouch of the abdominal cavity. Usually, the tumor presents as a paratesticular mass, such as a hydrocele or intrascrotal mass of unknown origin. In Plas et al's review, the most frequently presented symptom was scrotal enlargement with hydrocele in over 55% of cases or a paratesticular mass in over 30% [4]. Bisceglia et al showed that a hydrocele was positive in 26 out of 60 cases, a scrotal mass in 22 cases, and both symptoms in 3 cases [6]. In contrast, primary tumors arising from the spermatic cord [5] were very rare. Tumors arising from the peritoneal mesothelium of an inguinal hernia sac, which are included among paratesticular MMs, account for less than 10% of total cases [6].
A history of direct occupational or familial asbestos exposure is found in around 35% to 40% of cases [4]. Those diagnosed between the ages of 20 and 40 years usually have a history of childhood exposure. The long latency period between exposure to asbestos and the onset of MM, which can range from 15 to 60 years, explains why mortality rates from mesothelioma have continued to rise. The diagnosis of mesothelioma is directly attributable to occupational asbestos exposure; however, there is evidence that mesothelioma may result from both para-occupational exposure, such as women having laundered their husband's overalls, and environmental exposure [7].
The combination of an accurate history, examination, radiology, and the acquisition of pathology is essential in the diagnosis of mesothelioma. On clinical examination, the tumor is mostly misinterpreted as a testicular tumor or hydrocele, but other preoperative diagnoses include diverse entities such as epididymitis, scrotal or inguinal hernia, spermatocele, and adenomatoid tumor [4,5]. Hence, correct preoperative diagnoses are made only in very few cases.
Radiological imaging is essential for the diagnosis, staging, and management of mesothelioma. X-ray, CT, magnetic resonance imaging (MRI), and positron emission tomography (PET) have all been used to evaluate the disease [7].
The correct diagnosis is usually determined on postoperative pathologic examination. Microscopically, a paratesticular MM is most often a pure epithelial type or mixed; only 2 cases of a pure sarcomatous spindle cell type have been reported [6]. The architectural pattern is usually papillary or tubulo-papillary. Radical orchiectomy with complete histologic examination to exclude any foci of stromal invasion is mandatory for accurate diagnosis, and a close follow-up of the patient is required for an adequate duration. Immunohistochemically, mesotheliomas are positive for cytokeratin, cytokeratin 7, epithelial membrane antigen, thrombomodulin, and calretinin, whereas they are negative for cytokeratin 20. Carcinoembryonic antigen and Leu-M1 may sometimes give an inappropriate hyaluronidase-sensitive staining. Vimentin may provide variable outcomes, ranging from negative to focally or diffusely positive results.
The differential diagnosis of paratesticular mesothelioma mostly includes both benign neoplastic and nonneoplastic mesothelial lesions arising in this site, such as adenomatoid tumors, cysts of the tunica albuginea, cysts of the testicular parenchyma, hydrocele-associated reactive mesothelial hyperplasia, and nonmesothelial tumors of diverse histogenetic origin [6].
Mesotheliomas are difficult to manage, and no clear guidelines exist for management. First-line surgical treatment is inguinal radical orchiectomy.
Paratesticular MMs are aggressive neoplasms capable of widespread local involvement as well as lymphatic and hematogeneous metastases [4,8,9]. Recurrence occurs in 60% of patients within 2 years, with a mortality rate of 30% after a median survival of 24 months [4,8]. Surgical options in the inguino-scrotal area as well as an early tumor stage at the time of diagnosis are likely to result in a slightly better prognosis. The most important prognostic factor for survival is the age of patient at the time of diagnosis, with significantly better survival in patients younger than 60 years of age [4,8,10].
A more limited conservative approach should be discouraged because it is almost always complicated by local recurrences. CT study is recommended for detection of retroperitoneal lymph nodal spread, which is found in 15% of cases [8]. Para-aortic lymph nodes are the most frequently and primarily involved, whereas pelvic (iliac and obturator) nodes are usually involved in more advanced stages. Radiation therapy and chemotherapy have been used [10], but showed minimal effectiveness. Early accurate, preoperative diagnosis remains the sole favorable factor for more effective treatment and control of this disease.
Despite their rare occurrence in the spermatic cord, paratesticular mesothelioma needs to be suspected, especially in patients with a history of asbestos exposure. In addition, after the diagnosis is confirmed as MM, a more aggressive management including a second-look operation with pelvic lymphadenectomy or adjunctive chemotherapy may be required.
Figures and Tables
FIG. 1
Computed tomography scan showing a 3.2 cm lobulated mass in the left inguinal area (A) and a hydrocele in the left scrotum (B).
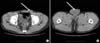
FIG. 2
On gross examination, the left orchiectomy specimen was composed of a 5.0×6.0 cm sized testis, attached to a 12 cm length spermatic cord and detached tunica vaginalis hydrocele sac. At the spermatic cord, there was a nodular mass measuring 5.0×3.2×2.5 cm. The outer surface of the mass was relatively well capsulated and the cut surface was shiny and whitish.

References
1. Park HM, Kim JH, Bae SG, Kwon TK, Chung SK. A case of malignant mesothelioma of tunica vaginalis. Korean J Urol. 1997. 38:1132–1134.
2. Lee SC, Lee JK. A case of primary malignant mesothelioma of tunica vaginalis testis. Korean J Urol. 1991. 32:843–845.
3. Shin TK, Lee TY, Park MH. Mesothelioma of the tunica vaginalis of the spermatic cord with coincidental finding renal cell carcinoma. Korean J Urol. 1995. 36:1142–1146.
4. Plas E, Riedl CR, Pflüger H. Malignant mesothelioma of the tunica vaginalis testis: review of the literature and assessment of prognostic parameters. Cancer. 1998. 83:2437–2446.
5. Perez-Ordonez B, Srigley JR. Mesothelial lesions of the paratesticular region. Semin Diagn Pathol. 2000. 17:294–306.
6. Bisceglia M, Dor DB, Carosi I, Vairo M, Pasquinelli G. Paratesticular mesothelioma. Report of a case with comprehensive review of literature. Adv Anat Pathol. 2010. 17:53–70.
7. Moore AJ, Parker RJ, Wiggins J. Malignant mesothelioma. Orphanet J Rare Dis. 2008. 3:34.
8. García de Jalón A, Gil P, Azúa-Romeo J, Borque A, Sancho C, Rioja LA. Malignant mesothelioma of the tunica vaginalis. Report of a case without risk factors and review of the literature. Int Urol Nephrol. 2003. 35:59–62.
9. Goel A, Agrawal A, Gupta R, Hari S, Dey AB. Malignant mesothelioma of the tunica vaginalis of the testis without exposure to asbestos. Cases J. 2008. 1:310.
10. Hassan R, Alexander R. Nonpleural mesotheliomas: mesothelioma of the peritoneum, tunica vaginalis, and pericardium. Hematol Oncol Clin North Am. 2005. 19:1067–1087.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download