Abstract
Purpose
The objective of this study was to investigate the diagnostic accuracy of multi-detector computerized tomography urography (MDCTU) for the detection of bladder tumors.
Materials and Methods
We retrospectively reviewed the medical records of 143 patients who were scanned by use of 64-channel MDCTU and who underwent cystoscopy due to painless hematuria or a clinical suspicion of bladder tumor. We examined the accuracy of MDCTU for the detection of bladder tumors by comparing the results obtained by MDCTU with those obtained by cystoscopy. The associations between tumor characteristics, frequency of transurethral resection (TUR), and bladder volume and detectability of bladder tumors on MDCTU were also analyzed.
Results
Of 143 patients, 50 patients had a history of urothelial carcinomas. In these patients, the sensitivity and specificity of MDCTU were 60.0% and 80.0%, respectively. In 93 patients without previous urothelial carcinomas, the sensitivity and specificity of MDCTU were 86.7% and 96.8%, respectively. Falsely diagnosed cases had a smaller distended bladder volume (p=0.014) and a smaller tumor size (p=0.022) than did true diagnosed cases. The false-negative rate increased when the bladder tumor was located at the bladder neck. In the univariate analysis, the tumor location, size, frequency of TUR, bladder volume, and initial hematuria were associated with detectability by MDCTU (p<0.05).
Conclusions
To improve the accuracy of MDCTU for diagnosing bladder tumors, bladder filling is recommended. Thus, cystoscopy should be considered as a standard diagnostic tool for bladder tumors even in patients with normal MDCTU results, especially in the evaluation of recurrent, bladder neck-located, small, or sessile bladder tumors.
Traditionally, physicians use imaging modalities, such as excretory urography, to diagnose bladder tumors preoperatively. Currently, abdominal ultrasonography (USG), computed tomography (CT), and magnetic resonance imaging (MRI) are also used. With excretory urography and USG, there are some limitations in visualizing the entire urinary tract. However, the latest technology, multi-detector computerized tomography urography (MDCTU), uses the newly developed MDCT, which emerged with the development of spiral CT technology and has the advantage of visualizing the entire urinary tract, including the renal pelvis, the ureter, the bladder, and the renal parenchyma [1-3].
MDCTU has recently been used in patients with hematuria for detecting urinary tract lesions. However, MDCTU can be performed more quickly than an so that bladder filling may be inadequate for the evaluation of bladder lesions. We therefore sought to determine the diagnostic accuracy of MDCTU for detecting bladder tumors and the factors affecting the detection rate by MDCTU.
The study was carried out in accordance with the Declaration of Helsinki, and informed consent was obtained from all subjects after they were provided a detailed description of the procedures.
We retrospectively reviewed the medical records of 323 patients who underwent MDCTU between January 2008 and May 2009. We enrolled patients who underwent MDCTU due to painless hematuria or as a follow-up study for urothelial cancer. Patients who were lost to follow-up, declined cystoscopy, or underwent transurethral surgery for clinically suspicious bladder tumors were excluded from the analysis. Additionally, patients with more than a 1-month interval from MDCTU to cystoscopy were excluded. One hundred eighty patients were excluded; 143 patients who underwent both MDCTU and cystoscopy were included in the final analysis. Bladder tumors were pathologically diagnosed by cystoscopic biopsy or transurethral resection (TUR). Patients were considered negative for bladder tumor if no tumor was detected cystoscopically or if the biopsy specimen was determined to be nonmalignant on histopathologic examination.
MDCTU was performed by using a 64-channel volume CT (Lightspeed VCT; GE Healthcare, Milwaukee, WI, USA). All patients drank 700 to 1,200 ml of water before imaging. All examinations were supplemented with 250 ml of intravenous saline infused by gravity after the administration of contrast medium. All patients were scanned in the supine position. A three-scan CT protocol included an unenhanced scan (5 mm slice thickness, 5 mm interval, pitch of 1, 120 kVp, and 170-280 mA) of the abdomen and pelvis, a nephrographic phase scan of the kidneys (3.75 mm slice thickness, 3.75 mm interval, pitch of 1,120 kVp, and 170-280 mA) 90 seconds after intravenous administration of 125 to 140 ml of iopromide (Ultravist 300; Berlex Laboratories, Madison, NJ, USA) at a rate of 3 ml/sec, and an excretory phase scan of the abdomen and pelvis (3.75 mm slice thickness, 3.75 mm interval, pitch of 1,120 kVp, and 170-280 mA) 10 minutes after the injection of contrast medium. Excretory phase scans were reconstructed in the coronal and sagittal planes with a 2 mm slice thickness. The average scanning time of MDCTU was 15 minutes.
CT scanning data of the excretory phase were sent to a workstation (Advantage Windows 4.2; GE Healthcare Technologies). Three-dimensional images with volume rendering (VR) and maximum-intensity-projection (MIP) techniques were reconstructed on the workstation. MDCTU images, including two-dimensional (axial, coronal, and sagittal images) and three-dimensional images, were interpreted by one radiologist (KJW) who was blinded to the clinical data (Fig. 1). The average interpretation time of the MDCTU, including two- and three-dimensional images, was 10 minutes.
Positive findings for bladder tumors were as follows: an intraluminal bladder mass, an asymmetrically thickened bladder wall, or a mass within the urethra.
Excluding the cases with asymmetrically thickened bladder walls, the longest diameter of the tumor was measured. In patients with multiple bladder tumors, the longest diameter of the largest tumor was measured. We calculated the capacity of the bladder by using the coronal, sagittal, and axial images of the excretory phase from the MDCTU by using an ellipsoid formula, as follows: front and rear diameter x width diameter x the head and tail diameter x π/6 [4].
We examined the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and factors associated with the detection of bladder tumors by MDCTU by comparing the results obtained by MDCTU with those obtained by cystoscopy. Also, we compared the clinical characteristics of the two subgroups of patients. The first subgroup consisted of falsely diagnosed patients who were diagnosed as false-negatives or false-positives by MDCTU, and the second subgroup consisted of patients in whom bladder tumors were correctly diagnosed by MDCTU.
The average age of the 143 patients was 63.38±12.13 years. There were 110 males (76.9%) and 33 females (23.1%) enrolled in the study. Ninety-three patients were evaluated for hematuria and 50 patients were evaluated as a follow-up for urothelial cancer.
In the 143 patients who underwent cystoscopy, 55 were diagnosed with bladder tumors (Table 1). On the basis of the 55 patients who were diagnosed with bladder tumors, the sensitivity, specificity, PPV, and NPV of MDCTU in all patients were 74.5%, 92.0%, 85.4%, and 86.3%, respectively.
In the 93 patients who underwent evaluation for hematuria, 30 were diagnosed with bladder tumors (Table 1); the sensitivity, specificity, PPV, and NPV of MDCTU were 86.7%, 96.8%, 92.9%, and 93.8%, respectively.
In the 50 patients who had a history of urothelial cancer, 25 were diagnosed with bladder tumors (Table 1); the sensitivity, specificity, PPV, and NPV of MDCTU were 60.0%, 80.0%, 75.0%, and 66.7%, respectively.
The sensitivity of MDCTU according to the frequency of TUR, tumor location, multiplicity of tumor, mass size, and distended bladder volume is shown in Table 1.
There were 14 false-negative patients in whom MDCTU did not detect a tumor. Of the 14 patients, 7 (50.0%) had bladder neck tumors (mean size, 1.10±0.50 cm; 0.5, 0.7, 1, 1, 1, 1.5, and 2 cm, respectively), 4 (28.6%) had intraluminal bladder tumors (mean size, 0.53±0.21 cm; 0.3, 0.5, 0.5, and 0.8 cm, respectively), 2 (14.3%) had flat-shaped bladder tumors, and 1 (7.1%) had a prostate urethral tumor (size, 1.5 cm) (Table 2).
On the basis of the MDCTU examinations, 7 patients were false-positives, and the bladder walls were partially thickened in all patients. These patients were diagnosed as having benign lesions (cystitis) on biopsy.
When comparing the 21 false-negative and false-positive patients and the 41 patients with MDCTU-diagnosed bladder tumors that were confirmed by biopsy, the former group of patients were more likely to have a history of urothelial cancers (71.4% vs. 36.6%; p=0.015) (Table 3), had smaller tumor sizes (0.89±0.49 cm vs. 1.95±1.39 cm; p=0.022) (Table 3), and had a less distended bladder volume (145.9±107.6 ml vs. 226.9±124.0 ml; p=0.014) (Table 3).
In the univariate analysis, the tumor location, size, frequencies of TUR, bladder volume, and initial hematuria were factors associated with the outcomes of MDCTU (Table 4). However, there were no significant factors in the multivariate analysis (data not shown).
The current study focused on the diagnostic efficiency of MDCTU for detecting bladder tumors and the factors affecting the sensitivity of MDCTU.
In evaluating hematuria and to establish an accurate diagnosis, physicians depend on various methods, which require exceptionally high clinical sensitivity and specificity. CT, with and without intravenous contrast, is being used more than excretory urography in the evaluation of hematuria. With computer-assisted reconstruction, longitudinal views of the urinary tract can now be made. MDCTU can promptly produce thin layers of the unenhanced, nephrographic, and excretory phases and can be used to reconstruct three-dimensional images (Fig. 1). Hence, MDTCU can generate further enhanced resolution of the pre-existing excretory urography [5]. Also, gases inside the organs, adjacent bone structures, and fecal material that hinder reading the results from excretory urography are overcome with MDCTU.
Kim et al reported the sensitivity and specificity of MDCT in diagnosing bladder cancer to be 89% and 95%, respectively [6]. According to several reports that examined the results of MDCTU in diagnosing bladder cancer, the sensitivity varied from 64 to 96% and the specificity also varied from 89 to 99% [7-10]. In our study, the sensitivity and specificity of MDCTU in screening examinations for initial hematuria were 86.7% and 96.8%; however, as a follow-up for urothelial cancer, MDCTU had a sensitivity and specificity of 60.0% and 80.0%, respectively, which is lower in patients with a history of urothelial cancer. This is probably because, in contrast with the initial hematuria patients, the follow-up patients had smaller tumors and had inflammatory changes to the bladder wall as a result of previous treatment.
We also analyzed the sensitivity of MDCTU according to the frequency of TUR, tumor multiplicity, size, location, and distended bladder volume. Jinzaki et al reported that the sensitivity of MDCT in distinguishing bladder tumors that were <0.5 cm in size was 58%, whereas the sensitivity for tumors between 0.5 and 1 cm in size was 94%; tumors that were >1 cm in size had a 100% sensitivity rate. In our study, the sensitivity of sessile tumors was 81.8%, and that of tumors that were <1 cm was 33%; tumors that were ≥1 cm in size had an 82.8% sensitivity rate compared with tumor size <1 cm, and sessile tumor and tumor size ≥1 cm were factors affecting positive outcomes [11]. Discrepancies between Jinzaki's results and ours were probably because our number of patients with tumors <1 cm or distended bladder volume was smaller than in Jinzaki's report.
The location of tumors may also affect the sensitivity of MDCTU. When we analyzed sensitivity stratified by tumor location, bladder neck tumors showed a low sensitivity (36%). Because of the effect of prostatic enlargement, partial opacification [with fluid-fluid (contrast) level] of the bladder may make bladder neck tumors more difficult to detect by MDCTU compared with intravesical tumors.
The frequency of TUR is also related to the accuracy of MDCTU. It was shown in patients with urothelial cancer previously treated by several TURs that the sensitivity of MDCTU was lower because the previous transurethral procedures led to injury or inflammation of the bladder mucosa.
Another important factor in diagnosing bladder tumors with MDCTU is distended bladder volume. In the current study, the sensitivity of MDCTU was increased with increasing distended bladder volume, and true-positive patients had an acceptable bladder volume (227 ml). The average bladder volume in patients who were diagnosed as false-negative and false-positive was 146 ml, which is unacceptable. It is not surprising to miss the diagnosis of a bladder tumor in a poorly distended bladder. If the bladder is less distended, the bladder wall more thickened, and small tumors less protruded, this may affect the false-positive rate. Also, cases of severe bladder trabeculations may preclude detection of tumors in this situation.
Shimizu et al reported that when diagnosing gastric cancer with MDCT and using the water-filling method, having the patient drink 300 to 600 ml of water before the CT scan not only enhances the sensitivity but also provides information on the rate of gastric wall permeation [12]. Like the stomach, the bladder is a hollow organ in which distended bladder volume affects the diagnostic accuracy of MDCTU. To prevent inappropriate bladder distention, we previously checked virtual cystoscopy with MDCT by distension of the bladder with approximately 300 to 500 cc of room air through a urethral catheter after drainage of urine and showed sensitivity of 70% to 100% [13]. Therefore, when using MDCTU to examine the bladder, it is recommended to instruct the patients not to empty their bladder before the examination.
MDCTU has progressively become a powerful tool in the evaluation of patients presenting with painless hematuria, and during the past few years has been able to demonstrate a wide spectrum of diseases affecting the urinary tract. The reliable depiction of the entire urinary tract, including the renal collecting systems, the ureters, and the bladder, is possible, and an important advantage of the technique is its ability to detect uroepithelial malignancies.
The disadvantage of MDCTU or MDCT is a radiation exposure dose higher than that with excretory urography or conventional CT. To prevent this, there are some reports recommending that MDCTU be avoided in patients who are <40 years of age with a lower incidence of urothelial cancer or who are sensitive to radiation [14,15]. Additionally, many reports have focused on maintaining the image quality of MDCTU and lowering the radiation exposure dose at the same time [16,17].
Although our data showed that the overall sensitivity of MDCTU was not sufficiently high to replace cystoscopy within the diagnostic pathway, the high specificity enables an early diagnosis and treatment in patients with initial hematuria and in follow-up patients. Furthermore, because this study clinically applied MDCTU to patients with initial hematuria and to the follow-up of patients with urothelial cancer as opposed to only patients with tumors, this study appears to be more significant.
This study is not without limitations, including its retrospective nature and small number of cases; also, we did not perform any manipulations such as log-rolling or retrograde dye instillation to obtain adequate opacification of the urinary bladder in the excretory phase. The multivariate analysis did not show any independent factors affecting MDCTU outcomes (results not shown). Further prospective studies might be needed to obtain adequate opacification of the urinary bladder without additional radiation exposures and to verify the independent factors affecting positive MDCTU results.
The diagnostic accuracy of MDCTU is affected by a history of urothelial cancer, tumor location, size, and distended bladder volume. The sensitivity of MDCTU in patients with a history of urothelial cancer is lower than in patients with hematuria. The falsely-diagnosed cases were more likely to have a history of urothelial cancer, less distended bladder volume, and a smaller tumor size than did the true diagnosed cases. The false-negative rate is increased when the bladder tumor is located at the bladder neck. The accuracy of MDCTU for diagnosing bladder tumors depends on bladder distension; therefore, bladder filling is recommended.
Cystoscopy should be considered as a standard diagnostic tool for bladder tumors, even in patients with normal MDCTU, especially in the evaluation of recurrent tumors, those located at the bladder neck located, small tumors, or sessile bladder tumors.
Figures and Tables
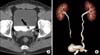 | FIG. 1Urothelial carcinomas of the bladder and left distal ureter. (A) Axial 2D and (B) 3D volume rendering (VR) CT urograms obtained during the excretory phase show an 11 mm mass (arrow) near the left ureterovesical junction and a 5 mm mass (arrowhead) in the left distal ureter in a 51-year-old man evaluated for gross hematuria. The masses were also detected during the cystoscopy performed after CT urography. |
References
1. Birnbaum BA, Jacobs JE, Ramchandani P. Multiphasic renal CT: comparison of renal mass enhancement during the corticomedullary and nephrographic phases. Radiology. 1996. 200:753–758.
2. Caoili EM, Cohan RH, Korobkin M, Platt JF, Francis IR, Faerber GJ, et al. Urinary tract abnormalities: initial experience with multi-detector row CT urography. Radiology. 2002. 222:353–360.
3. Chow LC, Sommer FG. Multidetector CT urography with abdominal compression and three-dimensional reconstruction. AJR Am J Roentgenol. 2001. 177:849–855.
4. Griffiths CJ, Murray A, Ramsden PD. Accuracy and repeatability of bladder volume measurement using ultrasonic imaging. J Urol. 1986. 136:808–812.
5. Akbar SA, Mortele KJ, Baeyens K, Kekelidze M, Silverman SG. Multidetector CT urography: techniques, clinical applications, and pitfalls. Semin Ultrasound CT MR. 2004. 25:41–54.
6. Kim JK, Park SY, Ahn HJ, Kim CS, Cho KS. Bladder cancer: analysis of multi-detector row helical CT enhancement pattern and accuracy in tumor detection and perivesical staging. Radiology. 2004. 231:725–731.
7. Sudakoff GS, Dunn DP, Guralnick ML, Hellman RS, Eastwood D, See WA. Multidetector computerized tomography urography as the primary imaging modality for detecting urinary tract neoplasms in patients with asymptomatic hematuria. J Urol. 2008. 179:862–867.
8. Chow LC, Kwan SW, Olcott EW, Sommer G. Split-bolus MDCT urography with synchronous nephrographic and excretory phase enhancement. AJR Am J Roentgenol. 2007. 189:314–322.
9. Anderson EM, Murphy R, Rennie AT, Cowan NC. Multidetector computed tomography urography (MDCTU) for diagnosing urothelial malignancy. Clin Radiol. 2007. 62:324–332.
10. Knox MK, Cowan NC, Rivers-Bowerman MD, Turney BW. Evaluation of multidetector computed tomography urography and ultrasonography for diagnosing bladder cancer. Clin Radiol. 2008. 63:1317–1325.
11. Jinzaki M, Tanimoto A, Shinmoto H, Horiguchi Y, Sato K, Kuribayashi S, et al. Detection of bladder tumors with dynamic contrast-enhanced MDCT. AJR Am J Roentgenol. 2007. 188:913–918.
12. Shimizu K, Ito K, Matsunaga N, Shimizu A, Kawakami Y. Diagnosis of gastric cancer with MDCT using the water-filling method and multiplanar reconstruction: CT-histologic correlation. AJR Am J Roentgenol. 2005. 185:1152–1158.
13. Jung SI, Kang TW, Shin SS, Kwon DD, Park K, Ryu SB. Usefulness of virtual cystoscopy using a 64-channel multidetector-row computed tomography scanner for detecting bladder tumors. Korean J Urol. 2007. 48:383–389.
14. Noroozian M, Cohan RH, Caoili EM, Cowan NC, Ellis JH. Multislice CT urography: state of the art. Br J Radiol. 2004. 77:S74–S86.
15. Van Der Molen AJ, Cowan NC, Mueller-Lisse UG, Nolte-Ernsting CC, Takahashi S, Cohan RH. CT urography: definition, indications and techniques. A guideline for clinical practice. Eur Radiol. 2008. 18:4–17.
16. Kemper J, Regier M, Bansmann PM, Begemann PG, Stork A, Nagel HD, et al. Multidetector CT urography: experimental analysis of radiation dose reduction in an animal model. Eur Radiol. 2007. 17:2318–2324.
17. Coppenrath E, Meindl T, Herzog P, Khalil R, Mueller-Lisse U, Krenn L, et al. Dose reduction in multidetector CT of the urinary tract. Studies in a phantom model. Eur Radiol. 2006. 16:1982–1989.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download