Abstract
Purpose
The aim of this study was to investigate the association of preoperative C-reactive protein (CRP) elevation and thrombocytosis with the prognosis of patients with non-metastatic renal cell carcinoma (RCC).
Materials and Methods
This was a retrospective review of the medical records of 177 patients (130 men and 47 women) with non-metastatic RCC who underwent a radical nephrectomy between March 2000 and May 2008 and for whom preoperative CRP and platelet data were available for analysis. Preoperative CRP elevation and thrombocytosis were compared with clinical and pathological variables.
Results
There were 38 patients with CRP elevation and 11 patients with thrombocytosis. The mean follow-up time was 48.3 months (median, 48.0; range, 13-111 months). Twenty-three patients (13.0%) developed metastases and six patients died during the follow-up period. CRP elevation was significantly correlated with anemia (p=0.001), T stage (p=0.004), grade (p=0.025), and metastasis (p<0.001). Thrombocytosis was significantly correlated with anemia (p=0.003), T stage (p=0.002), and metastasis (p=0.001). The univariate analysis identified anemia, CRP elevation, thrombocytosis, tumor histology subtype, tumor size, T stage, and grade as significant prognostic factors associated with recurrence-free survival, whereas the multivariate analyses showed that CRP elevation (p=0.033) and tumor size (p=0.007) were independent prognostic factors.
Conclusions
Preoperative CRP elevation and thrombocytosis were associated with a poorer prognosis and a higher recurrence rate in patients with non-metastatic RCC. Moreover, preoperative CRP elevation appeared to be an independent predictor of tumor recurrence and prognosis. Preoperative thrombocytosis, however, was not an independent prognostic factor for tumor recurrence and prognosis.
Renal cell carcinoma (RCC) is the most common primary malignant neoplasm of the kidney and accounts for about 3% of all adult neoplasms [1]. The incidence of RCC has been increasing during the past two decades in all age groups. The greatest increase has been in patients with localized tumors as a result of incidental detection, which is thought to be the result of more widespread use of abdominal ultrasound and computed tomography [2]. However, about 30% of patients with RCC already have metastatic disease at the time of diagnosis, and 10% to 14% of patients experience local recurrence or distant metastasis after nephrectomy despite a finding of pathologically confined disease at the time of nephrectomy [3-5].
Well-known pathological and clinical variables have been identified and sometimes integrated into nomograms to better predict disease recurrence, but with only partial success. With a better understanding of the biology of RCC, laboratory values and molecular markers are increasingly being investigated as potential predictive factors for RCC [6-8].
Disease progression appears to depend on a complex interaction between the tumor and host inflammatory responses. It is estimated that underlying infection and inflammatory responses are linked to 15% to 20% of all deaths from cancer worldwide [9]. The serum level of C-reactive protein (CRP), a nonspecific inflammatory acute-phase protein, is frequently increased in patients with metastatic RCC and is a predictor of prognosis [10].
Thrombocytosis is classified according to its etiology as primary or secondary. Primary thrombocytosis occurs in chronic myeloproliferative and in some myelodysplastic disorders [11]. However, secondary thrombocytosis has been reported with many malignant diseases; the survival of such patients has been reported to be significantly shorter [12,13]. The prognostic value of the platelet count has been studied in patients with localized and metastatic RCC; patients with thrombocytosis had a poorer survival than did patients with a normal platelet count [14,15].
The purpose of this study was to investigate the association of preoperative CRP elevation and thrombocytosis with the prognosis of patients with non-metastatic RCC.
The medical records of 177 patients with non-metastatic RCC who underwent a radical nephrectomy between March 2000 and May 2008 and for whom preoperative CRP and platelet levels were available for analysis were retrospectively reviewed. There were 130 men and 43 women, with a mean age of 53.5 years (range, 28-83 years). Preoperatively, all patients were evaluated with a physical examination, routine hematology and biochemical analysis, and radiology studies, including abdominal computed tomography and chest X-ray. The tumors were staged by using the 2002 TNM classification of the American Joint Committee on Cancer (AJCC) [16] and were graded according to Fuhrman's nuclear grading system [17]. All patients were evaluated postoperatively every 3 months for the first 2 years, every 6 months for the next 2 years, and yearly thereafter. CRP elevation was defined as a CRP level ≥0.8 mg/dl, and thrombocytosis was defined as a platelet count ≥380,000/µl in men and ≥370,000/µl in women according to the normal reference range in our hospital. Disease recurrence was defined as local failure in the tumor bed or regional lymph nodes or distant metastasis.
The chi-square test or Fisher's exact test was used to analyze the correlation between preoperative CRP elevation or thrombocytosis and the clinical and pathological variables, including age, gender, anemia, histology subtype, tumor size, T stage, nuclear grade, and metastasis during follow-up. Univariate and multivariate analyses were performed by using the log-rank test or the Cox proportional hazards regression model. For all tests, p<0.05 was considered to indicate statistically significant differences.
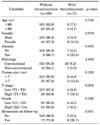
The clinical and pathological characteristics of the 177 patients with non-metastatic RCC are summarized in Table 1. The mean tumor size was 5.12 cm (median, 4.50; range, 1-22 cm). Regional lymph node dissection was performed in 24 patients who showed suspicious lymph node metastasis on computed tomography. However, none had a pathological diagnosis of lymph node metastasis. There were 38 patients (21.5%) with CRP elevations and 11 patients (6.2%) with thrombocytosis. Among patients with CRP elevation, 36 patients (94.7%) showed CRP normalization after surgery. The normalization occurred within 3 months of the radical nephrectomy. The mean follow-up period was 48.3 months (median, 48.0; range, 13-111 months). Twenty-three patients (13.0%) developed metastases and six patients died during the follow-up period.
Preoperative CRP elevation was significantly correlated with anemia (p=0.001), T stage (p=0.004), grade (p=0.025), and metastasis (p<0.001), but not with age, gender, tumor histology subtype, or tumor size (Table 2). Preoperative thrombocytosis was significantly correlated with anemia (p=0.003), T stage (p=0.002), and metastasis (p=0.001), but not with age, gender, tumor histology subtype, tumor size, or grade (Table 3). No significant correlation was found between preoperative CRP levels and platelet counts when analyzed by using Pearson's correlation coefficient (r=0.132, p=0.079).
Kaplan-Meier recurrence-free survival curves according to CRP level showed that the survival rate of patients with CRP elevation (CRP≥0.8 mg/dl) was significantly lower than that of patients with normal CRP levels (p<0.001) (Fig. 1). The univariate analysis identified anemia, CRP elevation, thrombocytosis, tumor histology subtype, tumor size, T stage, and grade as significant prognostic factors for recurrence-free survival, whereas the multivariate analysis showed that CRP elevation (p=0.033) and tumor size (p=0.007) were independent prognostic factors (Table 4).
CRP is an acute phase reactant that is produced exclusively by hepatocytes in response to cytokines such as interleukin (IL)-6, which are known mediators of ongoing inflammatory processes. An elevation of the serum concentration of this protein indicates the presence of acute inflammation, which is observed 8 to 12 hours after the onset of infection [18]. The relationship between cancer and CRP elevation is explained by overproduction of IL-6 by tumor cells [19]. Hepatic production of CRP is induced by cytokines such as IL-1, tumor necrosis factor (TNF), and primarily IL-6, which is frequently overproduced by the tumor cells themselves [18]. Experimental studies on RCC cell lines and expression studies of renal surgical specimens have shown that at least some renal tumors produce IL-6, which functions as an autocrine growth factor of the RCC [20]. Therefore, the presence of a systemic inflammatory response might be associated with aggressive RCC behavior.
The prognostic value of CRP levels has been reported in many recent studies of cancers, including those of the esophagus, ovaries, and colon [21-23]. However, the mechanism by which a systemic inflammatory response affects cancer-specific survival in patients with RCC is unclear. Hwang et al studied the relationship between CRP and survival in patients with RCC who underwent a radical nephrectomy [24]. They reported a statistically insignificant difference in the survival rates of patients with and without CRP elevation and concluded that CRP was not a significant prognostic factor for RCC when compared with tumor stage, grade, tumor size, and cell type. However, Komai et al demonstrated that the 5-year and 10-year disease-specific survival rates (75% and 30%, respectively) in patients with high CRP levels were significantly worse than the rates in patients with normal CRP levels (both 93%, p<0.001) and suggested that the preoperative CRP level is associated with poor survival in patients with localized RCC [25]. In addition, they reported that the CRP level is a useful preoperative screening tool in patients with localized RCC. Blay et al reported that pretreatment IL-6 and CRP were significantly associated with the effects of cytokine therapy and high IL-6 and that the CRP level indicated a poor response to cytokine therapy [26].
In the present study, preoperative CRP elevation was associated with a poorer prognosis and a higher recurrence rate in patients with non-metastatic RCC. This result confirms the importance of the preoperative CRP level as an independent prognostic factor for tumor recurrence and is consistent with the findings of earlier reports showing that a high CRP level is associated with poor survival in localized RCC [15,25].
Secondary or reactive thrombocytosis has been associated with tumor metastasis and poor survival in cancer patients. The pathophysiologic mechanism of reactive thrombocytosis may be tumor-associated elevation of circulating platelets by tumor-derived humoral factors such as IL-6 and macrophage colony-stimulating factor (M-CSF) that may be responsible for the development of cancer-related thrombocytosis [27]. IL-6 is a potent stimulator of megakaryocytopoiesis, and tumor cells have been shown to release IL-6 both in vitro and in vivo [27,28]. These observations suggest that IL-6 plays a role in the development of tumor-associated thrombocytosis.
Karakiewicz et al analyzed 1828 patients with RCC by univariable, multivariable, and predictive accuracy analyses with regard to RCC-specific mortality [29]. In that study, they showed that the addition of thrombocytosis to the base model (age, tumor size, TNM stage, ECOG-performance status, Fuhrman grade, and histology subtype) increased the predictive accuracy by only 0.3% (from 85.3% to 85.6%); these changes in predictive accuracy were not statistically significant. Consequently, they concluded that patients who presented with thrombocytosis did not have poorer prognosis than did their counterparts who did not exhibit these apparently unfavorable characteristics, as long as the effects of the TNM stage, histology, tumor grade, and ECOG-performance status were considered. However, many investigators have reported that thrombocytosis is related to a poor prognosis in patients with RCC. Göğüş et al reported the significance of thrombocytosis in localized RCC [14]. In that report, they showed that patients with thrombocytosis had a mean survival of 45.2 months compared with 76.6 months in patients without thrombocytosis (p=0.0002) and concluded that preoperative thrombocytosis is associated with a poorer survival than is a normal platelet count.
In the present study, we showed that preoperative thrombocytosis was significantly correlated with anemia, T stage, and metastasis. In addition, thrombocytosis was a significant prognostic factor associated with recurrence-free survival in the univariate analysis. Therefore, thrombocytosis was associated with a poorer prognosis and a higher recurrence rate in patients with non-metastatic RCC; these findings are consistent with those of previous studies [14,15].
Ito et al showed that preoperative CRP levels and platelet counts had a significant correlation and suggested that this was because the reactive thrombocytosis and CRP elevation in RCC were both caused by the production of inflammatory cytokines such as IL-6 [15]. As in the study by Ito et al, we analyzed the relationship between preoperative CRP and platelet count. However, we did not find a statistically significant association between preoperative CRP and platelet count (r=0.132, p=0.079). Therefore, the exact relationship between preoperative CRP and platelet count remains to be determined.
A limitation of this study is that several variables could not be considered in the current analysis. Nonetheless, all available variables were included, and our findings indicate that several known prognostic factors were valuable as prognostic factors. However, T stage and grade were not significant in the multivariate analysis. The reason for this result was, to some extent, too many patients in stage T1 rather than other T stages and a small number of patients with grade 4 compared with other grades according to the early detection of RCC nowadays.
Preoperative CRP elevation and thrombocytosis were associated with a poorer prognosis and a higher recurrence rate in patients with non-metastatic RCC. Moreover, preoperative CRP elevation was an independent predictor for tumor recurrence and prognosis. However, preoperative thrombocytosis was not an independent prognostic factor for tumor recurrence and prognosis.
Figures and Tables
 | FIG. 1Kaplan-Meier recurrence-free survival curves according to C-reactive protein (CRP) level. The survival rate of patients with CRP elevation (CRP≥0.8 mg/dl) was significantly lower than that of patients with a normal CRP level (p<0.001). |
TABLE 2
Relationship between preoperative C-reactive protein (CRP) level and clinicopathological variables in 177 patients with renal cell carcinoma

References
1. Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004. 54:8–29.
2. Gudbjartsson T, Thoroddsen A, Petursdottir V, Hardarson S, Magnusson J, Einarsson GV. Effect of incidental detection for survival of patients with renal cell carcinoma: results of population-based study of 701 patients. Urology. 2005. 66:1186–1191.
3. Ljungberg B, Alamdari FI, Stenling R, Roos G. Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur Urol. 1999. 36:565–569.
4. Levy DA, Slaton JW, Swanson DA, Dinney CP. Stage specific guidelines for surveillance after radical nephrectomy for local renal cell carcinoma. J Urol. 1998. 159:1163–1167.
5. Sorbellini M, Kattan MW, Snyder ME, Reuter V, Motzer R, Goetzl M, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005. 173:48–51.
6. Lee SE, Byun SS, Han JH, Han BK, Hong SK. Prognostic significance of common preoperative laboratory variables in clear cell renal cell carcinoma. BJU Int. 2006. 98:1228–1232.
7. Crispen PL, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED. Predicting disease progression after nephrectomy for localized renal cell carcinoma: the utility of prognostic models and molecular biomarkers. Cancer. 2008. 113:450–460.
8. Kwon WA, Park C, Kim EJ, Ha YS, Kim YJ, Yun SJ, et al. The relationship between RUNX3 inactivation and its pathological features in renal cell carcinoma. Korean J Urol. 2009. 50:432–438.
9. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001. 357:539–545.
10. Casamassima A, Picciariello M, Quaranta M, Berardino R, Ranieri C, Paradiso A, et al. C-reactive protein: a biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 based immunotherapy. J Urol. 2005. 173:52–55.
11. Mitus AJ, Schafer AI. Thrombocytosis and thrombocythemia. Hematol Oncol Clin North Am. 1990. 4:157–178.
12. Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996. 9:1826–1830.
13. Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002. 9:287–291.
14. Göğüş C, Baltaci S, Filiz E, Elhan A, Bedük Y. Significance of thrombocytosis for determining prognosis in patients with localized renal cell carcinoma. Urology. 2004. 63:447–450.
15. Ito K, Asano T, Yoshii H, Satoh A, Sumitomo M, Hayakawa M. Impact of thrombocytosis and C-reactive protein elevation on the prognosis for patients with renal cell carcinoma. Int J Urol. 2006. 13:1365–1370.
16. Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. American joint committee on cancer staging manual. 2002. 6th ed. New York: Springer-Verlag;323–328.
17. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982. 6:655–663.
18. Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992. 37:313–336.
19. Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, et al. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer. 2002. 86:1396–1400.
20. Miki S, Iwano M, Miki Y, Yamamoto M, Tang B, Yokokawa K, et al. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989. 250:607–610.
21. Guillem P, Triboulet JP. Elevated serum levels of C-reactive protein are indicative of a poor prognosis in patients with esophageal cancer. Dis Esophagus. 2005. 18:146–150.
22. Kodama J, Miyagi Y, Seki N, Tokumo K, Yoshinouchi M, Kobashi Y, et al. Serum C-reactive protein as a prognostic factor in patients with epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 1999. 82:107–110.
23. Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998. 176:335–338.
24. Hwang IC, Chang SG. Clinical significance of serum C-reactive protein in patients with renal cell carcinoma. Korean J Urol. 1999. 40:864–868.
25. Komai Y, Saito K, Sakai K, Morimoto S. Increased preoperative serum C-reactive protein level predicts a poor prognosis in patients with localized renal cell carcinoma. BJU Int. 2007. 99:77–80.
26. Blay JY, Negrier S, Combaret V, Attali S, Goillot E, Merrouche Y, et al. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992. 52:3317–3322.
27. Ramakrishnan S, Xu FJ, Brandt SJ, Niedel JE, Bast RC Jr, Brown EL. Constitutive production of macrophage colony-stimulating factor by human ovarian and breast cancer cell lines. J Clin Invest. 1989. 83:921–926.
28. Hollen CW, Henthorn J, Koziol JA, Burstein SA. Elevated serum interleukin-6 levels in patients with reactive thrombocytosis. Br J Haematol. 1991. 79:286–290.
29. Karakiewicz PI, Trinh QD, Lam JS, Tostain J, Pantuck AJ, Belldegrun AS, et al. Platelet count and preoperative haemoglobin do not significantly increase the performance of established predictors of renal cell carcinoma-specific mortality. Eur Urol. 2007. 52:1428–1436.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download