Abstract
In the present review article, we present an overview of beta-adrenoceptor (β-AR) subtype expression at the mRNA and receptor protein levels in the human detrusor, the in vitro and in vivo bladder function of the β3-AR, the in vivo effect of β3-AR agonists on detrusor overactivity in animal models, and the available results of clinical trials of β3-AR agonists for treating overactive bladder (OAB). There is a predominant expression of β3-AR mRNA in human bladder, constituting 97% of total β-AR mRNA. Also, functionally, the relaxant response of human detrusor to catecholamines is mainly mediated through the β3-ARs. Moreover, the presence of β1-, β2-, and β3-AR mRNAs in the urothelium and suburothelial layer of human bladder has been identified. Stimulation of urothelial β-ARs results in the release of nitric oxide and an unknown substance inhibiting detrusor contractions from the urothelium. Intravenous application of CL316,243, a selective β3-AR agonist, in rats selectively inhibits mechano-sensitive Aδ-fiber activity of the primary bladder afferents. A number of selective β3-AR agonists are currently being evaluated in clinical trials for OAB with promising preliminary results. In conclusion, the β3-AR agonists are the most notable alternative class of agents to antimuscarinics in the pharmacological treatment of OAB. The β3-AR agonists act to facilitate bladder storage function probably through at least two mechanisms: first, direct inhibition of the detrusor, and second, inhibition of bladder afferent neurotransduction.
Activation of beta-adrenoceptors (β-ARs) is involved in the bladder relaxation induced by sympathetic nerve activation during the storage phase. β-ARs were originally classified into two different subtypes, β1- and β2-ARs, on the basis of the different physiological effects of their ligands [1]. The classification of β-AR was modified following the discovery of a third β-AR primarily in rat adipose tissue in the 1980s [2]. Human β3-AR was cloned in 1989 and was initially identified in adipose tissue [3]. More recent reports indicate that β3-ARs are also expressed in human heart, gall bladder, gastrointestinal tract, prostate and urinary bladder detrusor, and brain [2]. In the late 1970s it was reported that isoproterenol-induced relaxation of human detrusor muscle is not blocked by practolol (a selective β1-AR antagonist) or butoxamine (a selective β2-AR antagonist), thus predicting the existence of a third β-AR subtype [4,5]. Later studies after the discovery of a new subtype (β3-AR) in various species to identify the functional involvement of β-AR subtypes in bladder relaxation demonstrated species differences. It has been suggested that the relaxation induced by β-AR agonists in detrusor muscle may be mediated mainly via β2-AR in rabbits [6-8], via both β2- and β3-AR in rats [6-10], via β2-AR and possibly β3-AR in pigs [11], but predominantly via β3-AR in dogs [8,10]. Which subtypes, then, are responsible for the relaxation of human detrusor?
The gene encoding the β3-AR has been cloned in several species: human [3], rat, mouse, bovine, monkey, and dog [12]. Human β3-ARs share 51% and 46% identity with the β1- and β2-AR amino acid sequences, respectively [3]. In humans, the gene is localized on chromosome 8. The rat and human β3-AR are 79% identical; the highest homology is in the transmembrane domains (94%), whereas the lowest homology is observed in the C-terminal tail and the third intracellular loop [2]. The β3-AR, together with β1- and β2-ARs, belongs to the G-protein coupled receptor family characterized by seven transmembrane domains of 22 to 28 amino acids, with three intracellular and three extracellular loops (Fig. 1) [2,3,12]. The N-terminal region of these receptors is extracellular and glycosylated, whereas the C-terminus is intracellular. The transmembrane domains, TM3, TM4, TM5, and TM6, are essential for ligand binding, whereas TM2 (which contains Asp83) and TM7 (which contains Tyr336) are involved in Gs activation [3]. The disulphide bond between Cys110 in the second and Cys189 in the third extracellular loops is essential for ligand binding and activity of the receptor. The Cys361 residue in the fourth intracellular domain is palmitoylated. Palmitoylation has been shown to mediate adenylate cyclase stimulation by agonist-bound receptor, possibly by promoting the insertion of several adjacent residues in the membrane and thus forming an additional intracellular loop resulting in an active conformation for G-protein coupling [2,13].
The expression of β1-, β2-, and β3-AR mRNA in the human bladder has been reported [14-17]. The presence of β3-AR mRNA in the human detrusor has also been confirmed in in situ hybridization studies [16]. It has also been demonstrated by use of a real-time quantitative polymerase chain reaction (PCR) that of all the β-AR mRNA in human bladder, 97% is represented by the β3-AR subtype and only 1.5% and 1.4% by the β1- and β2-AR subtypes, respectively [17]. Although a predominant expression of β3-AR mRNA was found in human detrusor tissue, evidence that the predominant subtype is the β3-AR cannot be drawn from this study in which only the receptor mRNA concentrations were determined without simultaneous determination of the receptor protein. Thus, whether the β3-AR is the most abundant subtype in human detrusor muscle remains to be elucidated [18].
The identification of β-ARs at the protein level is typically based on binding studies with various radioligands. Limited attempts have been made to identify the β-AR subtypes in the bladder by radioligand binding, demonstrating that sites in the rabbit and human bladder belong largely to the β2-AR subtype. However, the radioligands used in these studies have much lower affinity for β3- than for β1- and β2-ARs. Because of such problems, the currently available radioligand-binding techniques are probably inadequate to detect the presence of β3-ARs at the protein level because of the lack of selective ligands for β3-ARs [19,20].
In the human detrusor, Igawa et al originally reported that bupranolol (a nonselective β-AR antagonist) at a low concentration (10-8 M) did not inhibit isoproterenol-induced relaxation, but at higher concentrations (10-7-10-5 M), the drug caused a rightward shift of the concentration-relaxation curve for isoproterenol in a dose-dependent manner [21]. This suggests that the relaxation-induced adrenergic stimulation in the human detrusor is mediated mainly by β3-AR, rather than β1- or β2-AR, because bupranolol is known to have antagonistic action only on β1- and β2-ARs at low concentrations (nM), whereas at higher concentrations (µM) it also affects the β3-AR. In addition to the findings with bupranolol, neither dobutamine (a β1-AR agonist) nor procaterol (a β2-AR agonist) produced significant relaxation in the human detrusor [14,21]. On the other hand, the selective β3-AR agonists, BLR37,344, CL316,243, and CGP12,177A, all produced concentration-dependent relaxation [14,16]. Furthermore, isoproterenol-induced relaxation was inhibited by the selective β3-AR antagonists (SR58,894A), but not by the selective β1-AR antagonist (CGP20,712A), nor by the selective β2-AR antagonist (ICI118,551) (Fig. 2) [14]. In agreement with the predominant expression of β3-AR mRNA in the human bladder (see above), these findings indicate that the relaxation induced by β-adrenergic stimulation of the human detrusor is mediated mainly through β3-AR activation. Thus, this subtype is likely to be the most important for bladder relaxation in vitro.
Few studies have focused on possible changes in β-AR responsiveness in human detrusor in pathological conditions such as neurogenic bladder. A comparative study of neurogenic (low compliance or overactive) bladder preparations with controls was reported on the relaxant responses to isoproterenol (non-subtype-selective β-AR agonist) and three kinds of selective β3-AR agonists, BRL37,344, CL 316,243, and CGP 12,177. Agonist potency was similar in all three groups for each agonist, and maximum effects were also similar across the groups for the agonists, except for an increased effect of CGP 12,177 in low-compliance bladders [22]. These findings suggest that β3-AR-mediated relaxation can be preserved even in bladder tissues taken from patients with neurogenic low-compliant or overactive bladder (OAB). Nomiya and Yamaguchi compared bladder preparations taken from male patients with and without bladder outlet obstruction and found that potency and maximum effects of isoproterenol and L755,507 (the β3-AR agonist) were similar in both groups, although there were statistically significant differences in the abundance of β1-, β2-, or β3-adrenoceptor mRNA between the groups [17]. Taken together, even though the available human data were limited, it seems likely that the β-AR responsiveness can be largely preserved even in the human detrusor in states of bladder dysfunction.
The in vivo effects of β-AR agonists on bladder function have been studied in animal models. In urethane-anaesthetized rats, isoproterenol, the β2-agonist procaterol, and the β3-agonist CL316,243 dose-dependently decreased intravesical pressure in an isovolumetric condition. CL316, 243 had only a slight influence on blood pressure and heart rate, whereas isoproterenol and procaterol significantly affected cardiovascular function at the same dose range as that required to reduce bladder pressure. In cystometry experiments, CL316,243 (10 µg/kg i.v.) significantly prolonged the micturition interval and increased bladder capacity, but did not change the residual volume. On the other hand, procaterol (100 µg/kg i.v.) prolonged the micturition interval and increased bladder capacity but also increased residual volume [23]. In another study, both procaterol and CL316,243 reduced intravesical pressure in urethane-anaesthetized rats; the effect of procaterol was inhibited by ICI118,551 and the CL316,243 effect by the β3-AR antagonist L748,337, whereas neither antagonist affected the response to the other agonist [10]. In conscious, unrestrained rats, intravenous administration of procaterol did not affect voiding pressure relative to vehicle and had little effect on bladder capacity, whereas CL316,243 had no effect on bladder capacity but reduced voiding pressure [24]. These data suggest that both β2- and β3-adrenoceptors contribute to bladder relaxation in rats in vivo, which is consistent with the available in vitro data (see above).
It was shown that CL316,243 (β3-AR agonist) can improve detrusor overactivity at the doses studied without affecting the cardiovascular system or voiding function in rat experimental models associated with cerebral infarction [24], bladder outlet obstruction and intravesical instillation of acetic acid [25], and intravesical instillation of prostaglandin (PG) E2 [26], thus predicting the usefulness of β3-AR agonists in the treatment of OAB. The effect of CL316,243 was also investigated in a model of detrusor overactivity in spontaneously hypertensive rats (SHRs) [27]. CL316,243 inhibited bladder rhythmic contractions in SHRs, decreasing both frequency and amplitude of contraction. CL316,243 failed to reduce visceromotor reflex (VMR) or pressor responses to urinary bladder distension in either SHRs or normotensive control rats, which suggests that the β3-AR agonist inhibits bladder functions by acting directly on the detrusor. Together, these results suggest that the activation of β3-ARs increases bladder capacity without influencing bladder contraction or residual urine volume during the voiding phase in commonly used animal models of detrusor overactivity. This character distinguishes β3-AR agonists from antimuscarinic agents, which produce urinary retention by decreasing the intensity of detrusor contraction. With regard to why β3-AR agonists do not affect voiding function, the following mechanism may be considered. The activation of β3-AR decreases bladder smooth muscle tone through an increase in adenylate cyclase activity during the filling phase. During the voiding phase, acetylcholine released from parasympathetic nerves activates postjunctional muscarinic M2 receptors and inhibits the increased adenylate cyclase activity mediated by β3-ARs. At the same time, acetylcholine also stimulates muscarinic M3 receptors and activates the phosphatidylinositol-Ca2+ recruitment system, which preserves voiding bladder contractions independently [18,24,25,28-30]. Thus, under the administration of β3-AR agonists, at the beginning of the voiding phase, the relaxation of bladder smooth muscle may be canceled by muscarinic M2-receptor activation, but muscarinic M3-receptor-mediated bladder contraction can remain intact, suggesting in turn little risk of β3-AR agonists for causing the urinary retention noted with antimuscarinic agents. However, recent studies at least in the rat bladder have questioned cAMP-dependent routes as the exclusive mechanisms for triggering the β-AR-mediated relaxation [18,31,32]. Therefore, the in vivo effect of β3-AR agonists to increase bladder capacity without influencing bladder contraction cannot be simply explained [18]. Nevertheless, if this characteristic of β3-AR agonists is the case also in humans, the β3-AR agonists may have an advantage over antimuscarinics, especially in OAB patients with associated bladder outlet obstruction.
Otsuka et al confirmed the presence of β1-, β2-, and β3-AR in human urinary bladder urothelium by RT-PCR and immunohistochemistry [33]. It has been shown that activation of β-AR by isoproterenol in rat urothelial cells can release nitric oxide (NO) through an increase in intracellular Ca2+ by cAMP accumulation [34]. Activation of urothelial β-AR releases not only NO but also a urothelial-derived factor that inhibits contractions induced by carbachol in the pig detrusor [35]. The β-AR involved in the release of urothelial-derived inhibitory factor (UDIF) was recently determined to be a β3-subtype [36]. A similar UDIF released by β-AR activation in the human bladder urothelium is reported to inhibit the β-AR agonist-induced relaxation of the human detrusor smooth muscle [33].
The presence of all three β-AR subtypes has been demonstrated also in suburothelial interstitial cells of the human bladder by immunohistochemistry [37]. More recently, Limberg et al reported that in human female bladder, immunostaining for β1-, β2-, and β3-ARs was each more prominent in the urothelium than in the detrusor, with all receptors expressed in the same cell types, indicating co-expression of all three receptors throughout the urothelium in addition to the detrusor [38]. The authors demonstrated staining of all the three β-AR subtypes also in suburothelial myofibroblast-like cells, intramural ganglion cells, and in Schwann cells of intramural nerves, suggesting that activation of the myocyte receptor may be influenced by action on non-myocyte structures including the intramural ganglion cells and myofibroblasts. Aizawa et al reported, by use of a single-unit afferent activity monitoring procedure of the mechanosensitive primary bladder afferent nerves in the rat, that systemic activation of β3-ARs by intravenous administration of CL316,243 inhibits the mechanosensitive Aδ afferent fiber activity and PGE2-induced C-fiber hyperactivity [39]. This may imply that β3-AR agonists act as therapeutic agents for facilitating bladder storage function through at least two mechanisms: first, direct inhibition of the detrusor, and second, inhibition of bladder afferent neurotransduction.
BRL37344, CL316,243, and CGP-12,177A are representative β3-AR agonists that were optimized by using rodent β-ARs [19,40,41]. These compounds have lower potency for human β3-ARs than for rodent receptors, and they act as only partial agonists in humans [14,18-20,22,42]. Subsequent recognition of important pharmacological differences between rodent and human β3-ARs has led to the development of novel β3-AR agonists that are potent and highly selective toward human β3-ARs [19,30,42,43].
YM178, which was synthesized by Astellas Pharma Inc. (Ibaraki, Japan), showed highly selective agonist activity for human β3-AR over β1- or β2-AR. The agonistic potency of YM178 for human β3-ARs was 20 and 200 times that of BRL37344 and CL316,243, respectively [30]. In addition, the intrinsic activity of YM178 for human β3-ARs was higher than that of BRL37344 and CL316,243 [30]. In rat and human bladder strips precontracted with carbachol, YM 178 and isoproterenol concentration-dependently induced relaxation (Fig. 3), indicating full agonistic action for YM178 in both rat and human detrusor relaxation. In anaesthetized rats, YM 178, given intravenously at a dose of 3 mg/kg, decreased the frequency of rhythmic bladder contractions induced by intravesical filling with saline without suppressing its amplitude [30].
A recent study investigated the effects of another novel selective β3-AR agonist GW427353 (solabegron) on bladder function in dogs by use of in vitro and in vivo techniques. The relaxation evoked by this compound was attenuated by the nonselective β-AR antagonist bupranolol and SR 59230A (a selective β3-AR antagonist), but not by atenolol (a selective β1-AR antagonist) or ICI118,551 (a selective β2-AR antagonist). GW427,353 also increased the volume required to evoke micturition in anesthetized dogs following acetic acid-induced bladder overactivity without affecting voiding efficiency [44]. The effect of GW427,353 on human bladder strips was also reported [45]; the compound produced significant relaxation at concentrations of >10-7 M; isoproterenol produced a significant effect from 10-6 M, but otherwise both agonists had similar effects. GW427,353 at 10-6 M significantly reduced spontaneous activity within 10 min of incubation and at higher concentrations (>5x10-6 M) inhibited detrusor contractions evoked by electrical field stimulation [45].
Together, these results have encouraged the clinical development of β3-AR agonists for the treatment of OAB. Patients with OAB are usually treated with muscarinic receptor antagonists, but the side effects of those antagonists as well as their poor efficacy in some patients have stimulated interest in the development of new β3-AR subtype-selective agonists. In this context, β3-ARs are now considered an attractive target in the management of this clinical condition. The β3-AR agonists solabegron and YM178 are currently in Phase II and Phase III clinical trials, respectively, for the treatment of OAB [46].
Chapple et al reported the results of a phase IIA trial of YM178 in patients with OAB, which was clinical proof of a concept (Blossom) study conducted in several European countries [47]. The study enrolled 314 patients with OAB symptoms, and 262 patients were randomly assigned to 4 groups: placebo, YM178 100 mg bid, YM178 150 mg bid, and tolterodine 4 mg qd for a 4-week treatment period. The primary endpoint was to evaluate efficacy, and the primary efficacy variable was the change in the mean number of micturitions per 24 hours as recorded on a frequency/volume chart. Both the YM178 100 mg and 150 mg treatment groups showed significant improvements in the mean number of micturitions per 24 hours compared with the placebo group (-2.19 and -2.21 vs. -1.18, respectively). The change in mean volume voided was dose-dependently increased in the YM178 treatment groups, and the YM178 150 mg treatment group showed a significant increase in this variable compared with the placebo group. Urgency episodes per 24 hours significantly decreased in both the YM178 100 mg and 150 mg treatment groups compared with the placebo group. No severe adverse events were reported and treatment was generally well tolerated. A small, mean increase in pulse rate with YM178 150 mg (5 beats per minute) was demonstrated, but this was not associated with a clinically significant increase in adverse events such as tachycardia and palpitations. All together, this phase IIA trial of YM178 was the first proof of concept study for β3-AR agonists improving OAB symptoms.
Following this promising result, a phase IIB trial of YM178 for OAB was carried out in Europe [48]. This trial was a dose-ranging study of once-daily mirabegron (an extended release formula of YM178) in OAB patients. A total of 928 patients were randomly assigned in 5 study arms (placebo, mirabegron [YM178] 25 mg, 100 mg, 150 mg, and 200 mg qd, for a 12-week treatment period), and the primary endpoint was to evaluate the dose-response relationship on efficacy. The mean number of micturitions per 24 hours decreased dose-dependently, and the decreases were statistically significant with mirabegron 50 mg and over compared with placebo. The mean volume voided per micturition also increased dose-dependently, and the increases were significantly greater with mirabegron 50 mg and over compared with placebo. In total, 45.2% of patients experienced adverse events, and the incidence of adverse events was similar among all treatment groups (placebo 43.2% vs. mirabegron 43.8-47.9%). The overall discontinuation rate owing to adverse events was 3.2% (placebo 3.0% vs. mirabegron 2.4-5.3%). The most commonly reported class of adverse events considered to be treatment-related by the investigators was gastrointestinal disorders, including constipation, dry mouth, dyspepsia, and nausea. There was no patient-reported acute retention. No significant difference in ECG parameters between the groups was demonstrated. However, a small but significant increase in mean pulse rate was associated with mirabegron 100 mg and 200 mg (1.6 and 4.1 beats per minute, respectively), although this was not in accordance with an increase in cardiovascular adverse events.
Highly potent selective agonists for human β3-ARs have been developed. Although the available information on clinical trials of this class of agents for treating OAB is limited, some of these agents are expected to be the most notable alternative class to antimuscarinics in the pharmacological treatment of OAB. The β3-AR agonists can be used to treat OAB, especially in patients with benign prostatic hyperplasia, without increasing postvoid residuals, which is an advantage over antimuscarinics. These agents may be therapeutic candidates also for bladder hypersensitivity related to facilitation of C-fiber activity of the primary bladder afferents.
Figures and Tables
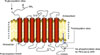 | FIG. 1Primary structure of the human beta3-adrenoceptor. Taken with permission from Ursino et al (2009) [2]. PKA: cAMP-dependent protein kinase, β-ARK: beta-adrenergic receptor kinase. |
 | FIG. 2Inhibition of isoprenaline-induced relaxation of human bladder detrusor by the β1-AR antagonist CGP 20,712, the β2-AR antagonist ICI 118,551, and the β3-AR antagonist SR 58,894. Taken with permission from Igawa et al (1999) [14]. |
 | FIG. 3Relaxing effect of isoproterenol, YM178, and CGP-12177A in rat (A) and human (B) bladder strips precontracted with carbachol. Taken with permission from Takasu et al (2007) [30]. |
References
1. Lands AM, Arnold A, McAuliff JP, Luduena FP, Brown TG Jr. Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967. 214:597–598.
2. Ursino MG, Vasina V, Raschi E, Crema F, De Ponti F. The beta3-adrenoceptor as a therapeutic target: current perspectives. Pharmacol Res. 2009. 59:221–234.
3. Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, et al. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989. 245:1118–1121.
4. Nergårdh A, Boréus LO, Naglo AS. Characterization of the adrenergic beta-receptor in the urinary bladder of man and cat. Acta Pharmacol Toxicol. 1977. 40:14–21.
5. Larsen JJ. Alpha and beta-adrenoceptors in the detrusor muscle and bladder base of the pig and beta-adrenoceptors in the detrusor muscle of man. Br J Pharmacol. 1979. 65:215–222.
6. Morita T, Iizuka H, Iwata T, Kondo S. Function and distribution of beta3-adrenoceptors in rat, rabbit and human urinary bladder and external urethral sphincter. J Smooth Muscle Res. 2000. 36:21–32.
7. Oshita M, Hiraoka Y, Watanabe Y. Characterization of beta-adrenoceptors in urinary bladder: comparison between rat and rabbit. Br J Pharmacol. 1997. 122:1720–1724.
8. Yamazaki Y, Takeda H, Akahane M, Igawa Y, Nishizawa O, Ajisawa Y. Species differences in the distribution of beta-adrenoceptor subtypes in bladder smooth muscle. Br J Pharmacol. 1998. 124:593–599.
9. Longhurst PA, Levendusky M. Pharmacological characterization of beta-adrenoceptors mediating relaxation of the rat urinary bladder in vitro. Br J Pharmacol. 1999. 127:1744–1750.
10. Takeda H, Matsuzawa A, Igawa Y, Yamazaki Y, Kaidoh K, Akahane S, et al. Functional characterization of beta-adrenoceptor subtypes in the canine and rat lower urinary tract. J Urol. 2003. 170:654–658.
11. Yamanishi T, Chapple CR, Yasuda K, Yoshida K, Chess-Williams R. The role of beta(3)-adrenoceptors in mediating relaxation of porcine detrusor muscle. Br J Pharmacol. 2002. 135:129–134.
12. Strosberg AD. Structure and function of the beta 3-adrenergic receptor. Annu Rev Pharmacol Toxicol. 1997. 37:421–450.
13. Strosberg AD, Pietri-Rouxel F. Function and regulation of the beta 3-adrenoceptor. Trends Pharmacol Sci. 1996. 17:373–381.
14. Igawa Y, Yamazaki Y, Takeda H, Hayakawa K, Akahane M, Ajisawa Y, et al. Functional and molecular biological evidence for a possible beta3-adrenoceptor in the human detrusor muscle. Br J Pharmacol. 1999. 126:819–825.
15. Fujimura K, Tamura K, Tsutsumi T, Yamamoto T, Nakamura K, Koibuchi Y, et al. Expression and possible functional role of the beta3-adrenoceptor in human and rat detrusor muscle. J Urol. 1999. 161:680–685.
16. Takeda M, Obara K, Mizusawa T, Tomita Y, Arai K, Tsutsui T, et al. Evidence for beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther. 1999. 288:1367–1373.
17. Nomiya M, Yamaguchi O. A quantitative analysis of mRNA expression of alpha 1 and beta-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J Urol. 2003. 170:649–653.
18. Yamaguchi O, Chapple CR. Beta3-adrenoceptors in urinary bladder. Neurourol Urodyn. 2007. 26:752–756.
19. Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006. 147:Suppl 2. S88–S119.
20. Vrydag W, Michel MC. Tools to study beta3-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2007. 374:385–398.
21. Igawa Y, Yamazaki Y, Takeda H, Akahane M, Ajisawa Y, Yoneyama T, et al. Possible beta 3-adrenoceptor-mediated relaxation of the human detrusor. Acta Physiol Scand. 1998. 164:117–118.
22. Igawa Y, Yamazaki Y, Takeda H, Kaidoh K, Akahane M, Ajisawa Y, et al. Relaxant effects of isoproterenol and selective beta3-adrenoceptor agonists on normal, low compliant and hyperreflexic human bladders. J Urol. 2001. 165:240–244.
23. Takeda H, Yamazaki Y, Akahane M, Igawa Y, Ajisawa Y, Nishizawa O. Role of the beta(3)-adrenoceptor in urine storage in the rat: comparison between the selective beta(3)-adrenoceptor agonist, CL316, 243,and various smooth muscle relaxants. J Pharmacol Exp Ther. 2000. 293:939–945.
24. Kaidoh K, Igawa Y, Takeda H, Yamazaki Y, Akahane S, Miyata H, et al. Effects of selective beta2 and beta3-adrenoceptor agonists on detrusor hyperreflexia in conscious cerebral infarcted rats. J Urol. 2002. 168:1247–1252.
25. Woods M, Carson N, Norton NW, Sheldon JH, Argentieri TM. Efficacy of the beta3-adrenergic receptor agonist CL-316243 on experimental bladder hyperreflexia and detrusor instability in the rat. J Urol. 2001. 166:1142–1147.
26. Takeda H, Yamazaki Y, Igawa Y, Kaidoh K, Akahane S, Miyata H, et al. Effects of beta(3)-adrenoceptor stimulation on prostaglandin E(2)-induced bladder hyperactivity and on the cardiovascular system in conscious rats. Neurourol Urodyn. 2002. 21:558–565.
27. Leon LA, Hoffman BE, Gardner SD, Laping NJ, Evans C, Lashinger ES, et al. Effects of the beta 3-adrenergic receptor agonist disodium 5-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino] propyl]-1,3-benzodioxole-2,2-dicarboxylate (CL-316243) on bladder micturition reflex in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2008. 326:178–185.
28. Igawa Y. Discussion: functional role of M(1), M(2), and M(3) muscarinic receptors in overactive bladder. Urology. 2000. 55:5A Suppl. 47–49.
29. Ehlert FJ, Ahn S, Pak KJ, Park GJ, Sangnil MS, Tran JA, et al. Neuronally released acetylcholine acts on the M2 muscarinic receptor to oppose the relaxant effect of isoproterenol on cholinergic contractions in mouse urinary bladder. J Pharmacol Exp Ther. 2007. 322:631–637.
30. Takasu T, Ukai M, Sato S, Matsui T, Nagase I, Maruyama T, et al. Effect of (R)-2-(2-aminothiazol-4-yl)-4'-{2-[(2-hydroxy-2-phenylethyl) amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther. 2007. 321:642–647.
31. Uchida H, Shishido K, Nomiya M, Yamaguchi O. Involvement of cyclic AMP-dependent and -independent mechanisms in the relaxation of rat detrusor muscle via beta-adrenoceptors. Eur J Pharmacol. 2005. 518:195–202.
32. Frazier EP, Mathy MJ, Peters SL, Michel MC. Does cyclic AMP mediate rat urinary bladder relaxation by isoproterenol? J Pharmacol Exp Ther. 2005. 313:260–267.
33. Otsuka A, Shinbo H, Matsumoto R, Kurita Y, Ozono S. Expression and functional role of beta-adrenoceptors in the human urinary bladder urothelium. Naunyn Schmiedebergs Arch Pharmacol. 2008. 377:473–481.
34. Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, et al. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002. 22:8063–8070.
35. Murakami S, Chapple CR, Akino H, Sellers DJ, Chess-Williams R. The role of the urothelium in mediating bladder responses to isoprenaline. BJU Int. 2007. 99:669–673.
36. Masunaga K, Chapple CR, McKay NG, Yoshida M, Sellers DJ. The β3-adrenoceptor mediates the inhibitory effects of β-adrenoceptor agonists via the urothelium in pig bladder dome. Neurourol Urodyn. 2010. 29:1320–1325.
37. Otsuka A, Matsumoto R, Shinbo H, Imanishi T, Kurita Y, Ozono S. Distribution of beta-adrenoceptor subtypes in suburothelial interstitial cells in the human urinary bladder. Neurourol Urodyn. 2009. 28:Suppl. 871–872. abstract 225.
38. Limberg BJ, Andersson KE, Aura Kullmann F, Burmer G, de Groat WC, Rosenbaum JS. β-adrenergic receptor subtype expression in myocyte and non-myocyte cells in human female bladder. Cell Tissue Res. 2010. 342:295–306.
39. Aizawa N, Igawa Y, Nishizawa O, Wyndaele JJ. Effects of CL316,243, a beta 3-adrenoceptor agonist, and intravesical prostaglandin E2 on the primary bladder afferent activity of the rat. Neurourol Urodyn. 2010. 29:771–776.
40. Arch JR, Ainsworth AT, Cawthorne MA, Piercy V, Sennitt MV, Thody VE, et al. Atypical beta-adrenoceptor on brown adipocytes as a target for anti-obesity drugs. Nature. 1984. 309:163–165.
41. Dolan JA, Muenkel HA, Burns MG, Pellegrino SM, Fraser CM, Pietri F, et al. Beta-3 adrenoceptor selectivity of the dioxolane dicarboxylate phenethanolamines. J Pharmacol Exp Ther. 1994. 269:1000–1006.
42. Michel MC, Ochodnicky P, Summers RJ. Tissue functions mediated by beta(3)-adrenoceptors-findings and challenges. Naunyn Schmiedebergs Arch Pharmacol. 2010. 382:103–108.
43. Hu B, Jennings LL. Orally bioavailable beta 3-adrenergic receptor agonists as potential therapeutic agents for obesity and type-II diabetes. Prog Med Chem. 2003. 41:167–194.
44. Hicks A, McCafferty GP, Riedel E, Aiyar N, Pullen M, Evans C, et al. GW427353 (solabegron), a novel, selective beta3-adrenergic receptor agonist, evokes bladder relaxation and increases micturition reflex threshold in the dog. J Pharmacol Exp Ther. 2007. 323:202–209.
45. Biers SM, Reynard JM, Brading AF. The effects of a new selective beta3-adrenoceptor agonist (GW427353) on spontaneous activity and detrusor relaxation in human bladder. BJU Int. 2006. 98:1310–1314.
47. Chapple CR, Yamaguchi O, Ridder A, Liehne J, Carl S, Mattiasson A, et al. Clinical proof of concept study (Blossom) shows novel β3 adrenoceptor agonist YM178 is effective and well tolerated in the treatment of symptoms of overactive bladder. Eur Urol. 2008. Suppl 7. 239.
48. Chapple C, Wyndaele JJ, van Kerrebroeck P, Radziszewski P, Dvorak V, Boerrigter P. Dose-ranging study of once-daily mirabegron (YM178), a novel selective β3-adrenoceptor agonist, in patients with overactive bladder (OAB). Eur Urol. 2010. Suppl 9. 249.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download