Abstract
Over the past two decades, laparoscopic and robotic surgery in children has been described as a viable minimally invasive alternative to open surgery for many pediatric urologic conditions. With the goal of reducing the morbidity associated with open surgery, minimally invasive surgery in children is increasingly being performed as laparoscopic and robotic patients appear to be experiencing shorter hospital stays, decreased pain medication requirements, and the potential for improved cosmesis. This article provides an overview of the existing literature in laparoscopic and robotic-assisted laparoscopic urologic surgery in children. Laparoscopic and robotic-assisted laparoscopic surgery appears to be safe and effective in children for a wide range of ablative and reconstructive procedures. Conventional laparoscopic surgery is effective for ablative procedures, while robotic surgery may be ideally suited for reconstructive cases requiring advanced suturing and dissection. Overall, more prospective studies are needed to study the long-term outcomes of minimally invasive surgery in pediatric patients, and the appropriate use of the available technology.
Go to : 
Laparoscopy has successfully been applied to a wide range of urologic procedures in adults and has led to reductions in the morbidity of surgical incisions, length of hospital stay, and length of convalescence. Since the initial description of diagnostic laparoscopy by Cortesi et al in 1976 for the diagnosis of a nonpalpable undescended testicle, laparoscopy has been widely used for many procedures in the field of pediatric urology [1]. Modifications of adult laparoscopic techniques for pediatric patients have resulted in the successful application of laparoscopic surgery in children for a wide range of procedures that continue to grow in number, such as laparoscopic nephrectomy and partial nephrectomy [2]. However, the application and development of minimally invasive techniques in pediatric patients may be lagging behind that in adult patients because of the long-term success of open surgery and the milder postoperative morbidity seen in pediatric patients [3]. In addition, the widespread adoption of laparoscopic surgery for pediatric urology cases may be hindered by the limitations of current laparoscopic equipment, which is not ideally adapted to the smaller working spaces in children, as well as the significant learning curve associated with laparoscopic pediatric reconstructive procedures.
New technological advances, such as robot-assisted laparoscopic surgery, may help to broaden the availability of minimally invasive surgery for reconstructive cases in the field of pediatric urology. As one example, the Da Vinci Surgical System (Intuitive Surgical, Sunnyvale, USA) offers the benefits of a user-friendly interface, three-dimensional visualization, magnification, and EndoWrist™ instruments that provide improved dexterity and precise control to the surgeon for delicate reconstruction procedures in children. In particular, the range of instrument articulation with the EndoWrist™ instruments is especially valuable in cases that require extensive dissection and suturing. These technological advances allow surgeons to apply familiar open surgery techniques in a minimally invasive fashion in the pediatric patient. Other technological advances are still needed for pediatric minimally invasive procedures, especially if the specifications are primarily developed with the pediatric patient as the focus.
In this review, we describe the latest developments in the current literature in laparoscopic and robotic surgery in the field of pediatric urology. We also discuss the current trends and outcomes in the application of laparoscopic and robotic surgery to this field.
Go to : 
A literature search of the PubMed database for all articles related to laparoscopic and robot-assisted laparoscopic surgery in the field of pediatric urology was performed. Key words used in our search included "laparoscopy," "robotics," "urology," and "pediatrics."
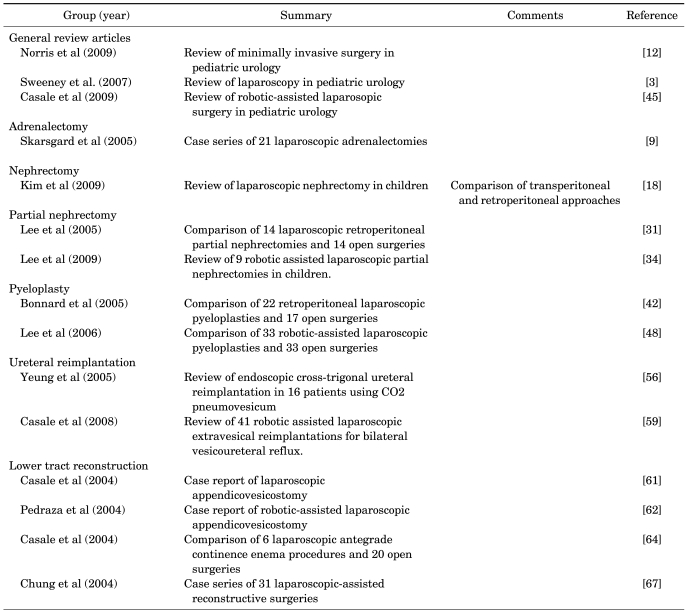
Laparoscopic adrenalectomy has been widely utilized in adult patients and is generally preferred over open surgery for the treatment of benign adrenal lesions in adults because of the decreased intra-operative blood loss, decreased analgesic requirements, shorter hospital stays, and superior cosmesis [4,5]. Even adrenal malignancies are completely resectable laparoscopically with similar outcomes to open surgery in terms of local recurrence rates and survival, although early reports were associated with a high risk of local recurrence [5-7].
In the pediatric population, the indications for adrenalectomy are similar to adults. However, a key difference in children is that the majority of pediatric adrenal lesions tend to be malignant, with neuroblastoma being the most common malignant adrenal lesion in children. This may have led to delays in the adaptation of minimally invasive techniques in this area.
Retroperitoneal and transperitoneal approaches for laparoscopic adrenalectomy have been reported previously in small pediatric clinical series. Mirallié et al described 6 transperitoneal and 2 retroperitoneal laparoscopic adrenalectomies in pediatric patients, with a mean tumor size of 4 cm [8]. Of note, 2 patients required conversion to open surgery. Skarsgard et al used a transperitoneal approach for 21 laparoscopic adrenalectomies in 20 patients with a mean operative time of 101 minutes and mean hospital length of stay of 1.5 days [9]. One patient required conversion to open adrenalectomy for treatment of an adrenal carcinoma with tumor thrombus extending into the renal vein. In pediatric patients with neuroblastoma, Iwanaka et al. compared the laparoscopic and open adrenalectomy techniques using adrenal biopsies and excisions in 37 patients [10]. Length of hospital stay and time to postoperative feeding and chemotherapy were noted to be shorter in the laparoscopic group than in the open surgery group. Other small series have described the use of laparoscopic partial adrenalectomies in the treatment of neuroblastoma, hereditary pheochromocytoma, and local tumor recurrence after open adrenalectomy [11,12].
Robotic-assisted laparoscopic adrenalectomy has been performed successfully in adults [13,14]. The noted benefits were the increased magnification and range of movement as compared to conventional laparoscopy, which aided in the dissection along major vessels and intraabdominal organs, as well as with isolation of the adrenal gland [4,15]. The use of robotic-assisted laparoscopy for adrenalectomy has not yet been described in children.
The most common indication for laparoscopic nephrectomy in the pediatric population is a nonfunctioning kidney due to obstructive uropathy, stone disease, vesicoureteral reflux, or multicystic dysplastic kidney [16]. Both transperitoneal and retroperitoneal approaches have previously been described. The transperitoneal approach offers the benefits of a larger working space and has been recommended when working bilaterally or when removing a multicystic dysplastic kidney [17,18]. However, both approaches have been shown to be safe with comparable outcomes and complication rates (retroperitoneal 4.3% and transperitoneal 3.5%) [18]. The most commonly reported complications include vascular injuries, bowel injuries, hematomas, urinomas, and port-site hernias [18].
El-Ghoneimi et al reported a series of 100 laparoscopic nephrectomies performed via the retroperitoneal approach with no open conversions [19]. The mean operative time was 97 minutes and the mean hospital stay was 1.9 days. In a retrospective study, Hamilton et al compared laparoscopic versus open nephrectomy/nephroureterectomy in 20 patients with nonfunctioning kidneys [20]. The mean operative time was noted to be greater in the laparoscopy group (175.6 versus 120.2 minutes), but the mean hospital stay (22.5 versus 41.3 hours) and qualitative analgesic demands were noted to be less in the laparoscopy group. In a recent study, Mahomed et al reported a series of 30 unilateral laparoscopic nephrectomies in which there were no open conversions. The mean operative time was 93 minutes and two-thirds of the patients were discharged home within 24 hours after surgery [21].
Laparoscopic surgery for pediatric renal neoplasms, such as renal cell carcinoma and Wilms tumor, continues to be controversial. There is currently no consensus on the limits of tumor size appropriate for surgery via laparoscopy in children for neoplastic conditions. For Wilms tumor, Duarte et al reported performing laparoscopic nephrectomies after neoadjuvant chemotherapy on 8 patients with Wilms tumors without complications [22]. However, more studies are needed to determine the safety and efficacy of laparoscopic surgery for renal malignancies in children.
Laparo-endoscopic single-site (LESS) nephrectomy for benign renal disease in children is the single-port modification of laparoscopic nephrectomy, by which surgery can be performed in a virtually scar-free fashion through a single incision in the umbilicus [23]. At our institution, in one of the largest known series to date (unpublished data), 11 pediatric patients ranging from infants to adolescents have undergone successful single-port laparoscopic nephrectomy for benign disease without conversion to open surgery. The LESS approach has been associated with improved cosmesis and a shorter recovery period compared with standard laparoscopic nephrectomy [23].
Robot-assisted laparoscopic nephrectomy can be performed, as with standard laparoscopic nephrectomy, by use of a transperitoneal or a retroperitoneal approach. Unfortunately, space requirements, especially in the infant population, often inhibit the retroperitoneal approach [24,25]. Whereas ablative procedures such as nephrectomy generally do not require the improved reconstructive capabilities associated with robotic surgery, these benign nephrectomies may be the ideal cases in the teaching setting for robotic procedures in preparation for more complex procedures.
The most common indication for partial nephrectomy or heminephrectomy in pediatric patients is a nonfunctioning upper- or lower-pole moiety in a duplicated system associated with ureterocele, ectopic ureter, or vesicoureteral reflux [26-28]. Duplicated systems in children have well-defined anatomic and vascular planes between the upper and lower systems, which decrease the risk of injury to the remaining moiety [29]. Similar to a laparoscopic simple nephrectomy, a transperitoneal or retroperitoneal approach may be used for laparoscopic partial nephrectomy. The benefits of a laparoscopic approach to partial nephrectomy are improved visualization from magnification, minimal blood loss, rapid recovery, and excellent cosmesis [30].
Horowitz et al reported a series of 14 transperitoneal laparoscopic partial nephrectomies with a mean operative time of 100 minutes, mean estimated blood loss of <30 ml, and mean hospital stay of 2.6 days. One complication of low hematocrit levels, managed conservatively without transfusion, was noted [30]. Lee et al reported a retrospective case-control study comparing an age-matched cohort of 28 pediatric patients undergoing open partial nephrectomy to patients undergoing retroperitoneal laparoscopic partial nephrectomy [31]. The laparoscopy group was found to have a shorter hospital stay (1.7 vs. 4.7 days) and lower narcotic requirements (0.44 vs. 1.53 mg/kg). Of note, a urinoma was noted as a complication in the laparoscopy group.
Complications have been noted in infants undergoing laparoscopic heminephrectomy. In an outcome analysis of 23 retroperitoneal laparoscopic heminephrectomies in children, Wallis et al reported on complete functional loss of the remaining ipsilateral moiety in 2 out of 5 infant patients [32]. It is unclear whether the loss of function was attributable to the laparoscopic retroperitoneal approach, to a greater risk of injury from the reduced working space compared with the transperitoneal approach, or to increased infant susceptibility to ischemic changes induced by retropneumoperitoneum [32]. Therefore, a transperitoneal approach may be more advantageous in the infant population [33].
Robot-assisted laparoscopic partial nephrectomy can also be performed via the transperitoneal and retroperitoneal approaches, although the benefits over conventional laparoscopic surgery remain to be proven, unless bladder reconstruction and ureteral reimplantation of the remaining moiety are necessary after ureterectomy [24]. Recently, Lee et al reported a retrospective series of robotic-assisted laparoscopic partial nephrectomy in 9 children [34]. All cases were successful with a mean operative time of 275 minutes, estimated blood loss of 49 ml, and mean hospital stay of 2.9 days. Postoperatively, the remaining renal moiety was normal on Doppler ultrasound in all patients. Complications were minimal, although a urinoma was reported in one patient.
Overall, several series have shown that both transperitoneal and retroperitoneal approaches for laparoscopic partial nephrectomy appear to be safe and effective. Operative times are comparable with those for open surgery, and improvements regarding postoperative pain, hospital stay, and cosmesis were seen compared with open surgery. Robot-assisted laparoscopic partial nephrectomy appears to be safe and effective; however, the advantages of the robot may best be seen in cases involving a significant reconstructive element.
Ureteropelvic junction obstruction (UPJO) is the most common obstructive uropathy found in children, and the gold standard for the treatment of UPJO is open pyeloplasty with success rates exceeding 90% [35,36]. Since the first laparoscopic pyeloplasty in a pediatric patient in 1993 [37], studies have shown that laparoscopic pyeloplasty in pediatric patients is associated with success rates comparable to those of open surgery [38,39].
Transperitoneal and retroperitoneal approaches have been used to perform laparoscopic pyeloplasties in children. The transperitoneal approach provides a larger working space and may facilitate suturing, whereas the retroperitoneal approach may make the dissection easier and reduce the risk of intra-abdominal injury [3]. Metzelder et al reported that transperitoneal laparoscopic pyeloplasty was safe and effective in all age groups in their series of 46 children [40]. In the largest series to date, Sweeny et al reported 107 laparoscopic Anderson-Hynes pyeloplasties, 4 laparoscopic Heineke-Milkulicz pyeloplasties, and 1 laparoscopic pyeloureterostomy with a success rate of 96.5% and one open conversion [3].
Outcomes from the retroperitoneal approach appeared to be similar to those of the transperitoneal approach. El-Ghoneimi et al reported a series of 22 retroperitoneal laparoscopic pyeloplasties with a mean operative time of 228 minutes and a mean hospital stay of 2.5 days [41]. Four patients did require conversion to open surgery because of the difficulty with completing the anastomosis laparoscopically. Bonnard et al published a retrospective comparison of retroperitoneal laparoscopic pyeloplasty (n=20) versus open pyeloplasty (n=17) [42]. The laparoscopy group was noted to have a shorter mean hospital stay (2.4 vs. 5 days) and a shorter time to cessation of pain medication requirements (1.9 vs. 3.22 days), but also a significantly longer mean operative time (219 vs. 96 minutes). Urine leakage was noted in both groups in 2 patients in each group, who were treated with ureteral stent placement or bladder drainage via a Foley catheter temporarily.
In general, however, laparoscopic pyeloplasty appears to be associated with a higher rate of secondary procedures. In their meta-analysis, Stefanie et al compared rates of reoperative intervention in open versus laparoscopic pyeloplasty. In the laparoscopy group, the reoperative intervention rate (7% vs. 3%) and redo pyeloplasty rate (4% vs. 2%) were higher in the laparoscopic pyeloplasty group than in the open pyeloplasty group [43].
Robot-assisted laparoscopic pyeloplasty in children is the most common procedure performed by use of the Da Vinci robot, and may be the best procedure to utilize the technical advantages of the robot to potentially decrease the higher rate of secondary procedures seen with conventional laparoscopic pyeloplasties [44,45]. In particular, the visual magnification and the range of instrument articulation with the EndoWrist™ instruments that assist with dissection and suturing provide improved dexterity and precise control to the surgeon for delicate reconstruction procedures in children, such as pyeloplasty [46]. Several studies have shown that robot-assisted laparoscopic pyeloplasty is safe and efficacious with success rates of approximately 95%, which is similar to the success rate of open surgery [46-48].
Olsen et al reported 67 robot-assisted retroperitoneal pyeloplasties in children with a median operative time of 143 minutes [49]. The 17.9% complication rate was noted to be similar to that of open surgery and included urinary tract infections in 2 patients, transient hematuria in 2 patients, displaced double-J ureteral stents in 3 patients, and nephrostomy tube placement in 4 patients [50]. One patient underwent conversion to open surgery because of a lack of space and camera movement limitations. Lee et al performed a retrospective case-control study of 33 transperitoneal robotic pyeloplasty patients and 33 open pyeloplasty patients [48]. The robotic surgery group had a shorter mean length of hospital stay (2.3 vs. 3.5 days) and a lower total narcotic requirement, although the mean operative time was higher in the robotic surgery group (219 vs. 181 minutes). However, the operative times did improve with experience to shorter times when compared with open surgery cases. Kutikov et al also reported on the successful use of robot-assisted laparoscopic pyeloplasties in 9 infants, although the small working spaces can be challenging [47].
Open ureterovesical reimplantation surgery has a long, favorable history in children with vesicoureteral reflux with success rates exceeding 95% and with minimal associated morbidity [3,12]. Laparoscopic ureterovesical reimplantation also appears to be safe and effective in children, especially with the use of the Lich-Gregoir extravesical technique, with shorter lengths of hospital stay, reduction in postoperative pain medication requirements, and improved cosmesis when compared with open surgery [51,52]. In one of the largest series published to date, Lakshmanan et al reported on successful laparoscopic extravesical ureteral reimplantation surgery in 47 children with 71 refluxing ureters [53]. Three ureteral injuries were noted, and the authors therefore stressed the importance of proper patient selection for this procedure. Age less than 4 years old (due to smaller working spaces) and the presence of concomitant ureteroceles or megaureters that require tapering may be possible contraindications for this approach.
Transvesical laparoscopic ureteral reimplantation, in which the bladder is insufflated with carbon dioxide and ureteral reimplantation is performed by using a combination of intravesical cystoscopy and laparoscopy, has also been reported with mixed results [54,55]. In their series of 29 children with 46 refluxing ureters undergoing transvesical laparoscopic ureteral reimplantation, Norris and Ost and Gatti et al reported success rates of 47% with the Gil-Vernet technique and 83% with the Cohen technique, with operative times nearly twice those of standard open techniques [12,54]. In more recent studies, a modified pneumovesicoscopic approach has been used, in which the laparoscope is placed transabdominally rather than transurethrally. In a series of 16 children with 23 refluxing ureters, Yeung et al performed Cohen cross-trigonal ureteral reimplantation by use of carbon dioxide pneumovesicum with a success rate of 96% [56]. The mean operative time was 136 minutes (112 and 178 minutes, unilateral and bilateral, respectively). In a retrospective series, Kutikov et al reviewed 32 children who underwent laparoscopic transvesical ureteral reimplantation. Cross-trigonal reimplantation was performed in 27 patients and a Glenn-Anderson reimplantation was performed in 5 patients with primary obstructing megaureter [57]. Success rates were noted to be 92.6% and 80%, respectively. Complications primarily occurred in patients aged 2 years and younger with bladder capacities less than 130 ml.
Robot-assisted laparoscopic surgery may help to improve the outcomes of minimally invasive surgery for vesicoureteral reflux in children. The increased magnification and EndoWrist™ instrument articulation may overcome the learning curve of the advanced laparoscopic skills necessary to perform suturing, knot tying, and advanced dissection required with laparoscopic ureteral reimplantation [12]. In addition, the improved dexterity with robotic surgery over conventional laparoscopy may allow utilization of surgical techniques that are similar to those of open surgical techniques [58].
The extravesical approach is the most commonly used approach to perform robot-assisted laparoscopic ureteral reimplantations, with similar steps to those used in the open and laparoscopic Lich-Gregoir techniques [24]. Casale et al performed a retrospective review of 41 patients undergoing robotic extravesical reimplantation for bilateral vesicoureteral reflux with a success rate of 97.6% and minimal complications [59]. They reported a mean operative time of 2.33 hours and average hospital length of stay of 26.1 hours.
Robot-assisted laparoscopic ureteral reimplantation has also been performed successfully via the pneumovesicoscopic approach and an intravesical cross-trigonal technique. As in conventional laparoscopic surgery, the procedure is not advocated in bladders smaller than 130 mL as seen on voiding cystourethrogram, because of working space limitations [25]. Peters et al reported a series of 6 children undergoing robot-assisted transvesical crosstrigonal reimplantation [60]. There were no open conversions in this group, and the mean duration of hospital stay ranged from 2 to 4 days. With one complication of a urinary leak, the authors noted that the pneumovesicoscopic approach is highly challenging, but can offer excellent visibility.
Slow, but steady, progress in the area of minimally invasive lower urinary tract reconstruction has been made over the past 2 decades, which can at least partially be attributed to the steep learning curve and the high potential for complications when working with the bowel in conjunction with the urinary tract. The recent literature is limited to case reports and small clinical series that describe the use of minimally invasive surgical techniques for the treatment of urinary and fecal incontinence. Laparoscopic Mitrofanoff appendicovesicostomy has been performed successfully by use of both conventional laparoscopic and robot-assisted laparoscopic approaches [61,62]. In cases that require significant suturing and dissection, robotic surgery appears to be helpful in addressing the technical demands of a particular procedure, such as improving the continence of the appendicovesicostomy anastomosis [24,63]. Casale et al compared laparoscopic antegrade continence enemas (ACE) in 6 patients with 20 open ACE patients and found no differences in operative times and complication rates, although the laparoscopy group reported decreased postoperative pain medication requirements and shorter hospital stays [64]. Other case reports of simultaneous robot-assisted laparoscopic Mitrofanoff and Malone ACE creation have also been reported [63,65].
The use of laparoscopy for bladder augmentation (enterocystoplasty) in patients with neurogenic bladders was initially reported by Docimo et al in 1995 with the use of a stomach segment [66]. Chung et al reported on the largest series to date of 31 patients undergoing laparoscopic- assisted surgery through a lower midline or Pfannenstiel incision, in which laparoscopic bladder augmentations were successfully performed with adequate postoperative capacity [67]. Lorenzo et al also reported performing a conventional laparoscopic ileal cystoplasty in a pediatric patient [68]. In general, adoption of laparoscopic techniques for bladder augmentation in children has been slow because of the technical complexity of the procedure with many of these patients having a history of previous abdominal surgery that may hinder the laparoscopic approach. In addition, the risk of ventriculoperitoneal shunt complications with intraperitoneal bowel leakage may be significant [68,69]. Robot-assisted laparoscopic surgery, with its technical advances that aid in delicate reconstruction procedures in children, may have a future role in these procedures.
Go to : 
An increasing body of literature is revealing that laparoscopic and robot-assisted urologic procedures are safe and effective in the pediatric population. As technique, experience, and laparoscopic equipment improve for pediatric patients, operative times should continue to decrease with expected improvements in perioperative outcomes. As the role of minimally invasive surgery becomes better defined in pediatric urologic surgery, we envision that conventional laparoscopy will likely become the standard of care in ablative procedures in pediatric urology, such as laparoscopic nephrectomy, whereas robot-assisted laparoscopic surgery will be the preferred modality for a minimally invasive approach to reconstructive procedures, such as pyeloplasty, which requires precise suturing. As the field of pediatric laparoscopic urology continues to mature, further trends toward "scarless" surgery, such as with LESS or single-site surgery, may continue as parents and patients seek minimally invasive options for their child's surgery. Future studies are necessary to determine the long-term safety, efficacy, and the appropriate indications of laparoscopic treatment modalities as they become available to more children.
Go to : 
References
1. Cortesi N, Ferrari P, Zambarda E, Manenti A, Baldini A, Morano FP. Diagnosis of bilateral abdominal cryptorchidism by laparoscopy. Endoscopy. 1976; 8:33–34. PMID: 16743.

2. Docimo SG, Peters CA. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Pediatric endourology and laparoscopy. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;p. 3907–3929.
3. Sweeney DD, Smaldone MC, Docimo SG. Minimally invasive surgery for urologic disease in children. Nat Clin Pract Urol. 2007; 4:26–38. PMID: 17211423.

4. Hyams ES, Stifelman MD. The role of robotics for adrenal pathology. Curr Opin Urol. 2009; 19:89–96. PMID: 19057223.

6. Kebebew E, Siperstein AE, Clark OH, Duh QY. Results of laparoscopic adrenalectomy for suspected and unsuspected malignant adrenal neoplasms. Arch Surg. 2002; 137:948–951. PMID: 12146996.

7. Moinzadeh A, Gill IS. Laparoscopic radical adrenalectomy for malignancy in 31 patients. J Urol. 2005; 173:519–525. PMID: 15643237.

8. Mirallié E, Leclair MD, de Lagausie P, Weil D, Plattner V, Duverne C, et al. Laparoscopic adrenalectomy in children. Surg Endosc. 2001; 15:156–160. PMID: 11285959.

9. Skarsgard ED, Albanese CT. The safety and efficacy of laparoscopic adrenalectomy in children. Arch Surg. 2005; 140:905–908. PMID: 16175699.

10. Iwanaka T, Arai M, Ito M, Kawashima H, Yamamoto K, Hanada R, et al. Surgical treatment for abdominal neuroblastoma in the laparoscopic era. Surg Endosc. 2001; 15:751–754. PMID: 11591983.

11. Cheng SP, Saunders BD, Gauger PG, Doherty GM. Laparoscopic partial adrenalectomy for bilateral pheochromocytomas. Ann Surg Oncol. 2008; 15:2506–2508. PMID: 18618188.

12. Norris RD, Ost MC. Evolution of laparoscopy in pediatric urology. Expert Rev Med Devices. 2009; 6:689–698. PMID: 19911879.

13. Desai MM, Gill IS, Kaouk JH, Matin SF, Sung GT, Bravo EL. Robotic-assisted laparoscopic adrenalectomy. Urology. 2002; 60:1104–1107. PMID: 12475680.

14. Undre S, Munz Y, Moorthy K, Martin S, Rockall T, Vale J, et al. Robot-assisted laparoscopic adrenalectomy: preliminary UK results. BJU Int. 2004; 93:357–359. PMID: 14764137.

15. Krane LS, Shrivastava A, Eun D, Narra V, Bhandari M, Menon M. A four-step technique of robotic right adrenalectomy: initial experience. BJU Int. 2008; 101:1289–1292. PMID: 18279451.

16. Casale P, Kojima Y. Robotic-assisted laparoscopic surgery in pediatric urology: an update. Scand J Surg. 2009; 98:110–119. PMID: 19799048.

17. Borzi PA, Yeung CK. Selective approach for transperitoneal and extraperitoneal endoscopic nephrectomy in children. J Urol. 2004; 171:814–816. PMID: 14713831.

18. Kim C, McKay K, Docimo SG. Laparoscopic nephrectomy in children: systematic review of transperitoneal and retroperitoneal approaches. Urology. 2009; 73:280–284. PMID: 18952262.

19. El-Ghoneimi A, Abou-Hashim H, Bonnard A, Verkauskas G, Macher MA, Huot O, et al. Retroperitoneal laparoscopic nephrectomy in children: At last the gold standard? J Pediatr Urol. 2006; 2:357–363. PMID: 18947636.

20. Hamilton BD, Gatti JM, Cartwright PC, Snow BW. Comparison of laparoscopic versus open nephrectomy in the pediatric population. J Urol. 2000; 163:937–939. PMID: 10688024.

21. Mahomed AA, Hoare C, Welsh F, Driver CP. A two-center experience with the exclusive use of laparoscopic transperitoneal nephrectomy for benign renal disease in children. Surg Endosc. 2007; 21:1532–1536. PMID: 17342559.

22. Duarte RJ, Dénes FT, Cristofani LM, Odone-Filho V, Srougi M. Further experience with laparoscopic nephrectomy for Wilms' tumour after chemotherapy. BJU Int. 2006; 98:155–159. PMID: 16831161.

23. Johnson KC, Cha DY, DaJusta DG, Barone JG, Ankem MK. Pediatric single-port-access nephrectomy for a multicystic, dysplastic kidney. J Pediatr Urol. 2009; 5:402–404. PMID: 19403335.

24. Casale P, Kojima Y. Robotic surgery in pediatric urology. AUA Update Series. 2009; 28:286–291.
26. Jednak R, Kryger JV, Barthold JS, González R. A simplified technique for upper pole heminephrectomy for duplex kidney. J Urol. 2000; 164:1326–1328. PMID: 10992406.
27. Plaire JC, Pope JC 4th, Kropp BP, Adams MC, Keating MA, Rink RC, et al. Management of ectopic ureters: experience with upper tract approach. J Urol. 1997; 158:1245–1247. PMID: 9258185.
28. Husmann DA, Ewalt DH, Glenski WJ, Bernier PA. Ureterocele associated with ureteral duplication and a non-functioning upper pole segment: management by partial nephrectomy alone. J Urol. 1995; 154:723–726. PMID: 7609163.
29. Jordan GH, Winslow BH. Laparoendoscopic upper pole partial nephrectomy with ureterectomy. J Urol. 1993; 150:940–943. PMID: 8345614.

30. Horowitz M, Shah SM, Ferzli G, Syad PI, Glassberg KI. Laparoscopic partial upper pole nephrectomy in infants and children. BJU Int. 2001; 87:514–516. PMID: 11298046.

31. Lee RS, Retik AB, Borer JG, Diamond DA, Peters CA. Pediatric retroperitoneal laparoscopic partial nephrectomy: comparison with an age matched cohort of open surgery. J Urol. 2005; 174:708–711. PMID: 16006955.

32. Wallis MC, Khoury AE, Lorenzo AJ, Pippi-Salle JL, Bägli DJ, Farhat WA. Outcome analysis of retroperitoneal laparoscopic heminephrectomy in children. J Urol. 2006; 175:2277–2282. PMID: 16697855.

33. Gonzalez R. Editorial comments. J Urol. 2010; Epub ahead of print.
34. Lee RS, Sethi AS, Passerotti CC, Retik AB, Borer JG, Nguyen HT, et al. Robot assisted laparoscopic partial nephrectomy: a viable and safe option in children. J Urol. 2009; 181:823–829. PMID: 19110277.

35. Persky L, Krause JR, Boltuch RL. Initial complications and late results in dismembered pyeloplasty. J Urol. 1977; 118:162–165. PMID: 875213.

36. Lowe FC, Marshall F. Ureteropelvic junction obstruction in adults. Urology. 1984; 23:331–335. PMID: 6369711.

38. Casale P, Grady RW, Joyner BD, Zeltser IS, Figueroa TE, Mitchell ME. Comparison of dismembered and nondismembered laparoscopic pyeloplasty in the pediatric patient. J Endourol. 2004; 18:875–878. PMID: 15659924.

39. Reddy M, Nerli RB, Bashetty R, Ravish IR. Laparoscopic dismembered pyeloplasty in children. J Urol. 2005; 174:700–702. PMID: 16006953.

40. Metzelder ML, Schier F, Petersen C, Truss M, Ure BM. Laparoscopic transabdominal pyeloplasty in children is feasible irrespective of age. J Urol. 2006; 175:688–691. PMID: 16407027.

41. El-Ghoneimi A, Farhat W, Bolduc S, Bagli D, McLorie G, Aigrain Y, et al. Laparoscopic dismembered pyeloplasty by a retroperitoneal approach in children. BJU Int. 2003; 92:104–108. PMID: 12823392.

42. Bonnard A, Fouquet V, Carricaburu E, Aigrain Y, El-Ghoneimi A. Retroperitoneal laparoscopic versus open pyeloplasty in children. J Urol. 2005; 173:1710–1713. PMID: 15821565.

43. Seixas-Mikelus SA, Jenkins LC, Williot P, Greenfield SP. Pediatric pyeloplasty: comparison of literature meta-analysis of laparoscopic and open techniques with open surgery at a single institution. J Urol. 2009; 182:2428–2432. PMID: 19765755.

44. Muneer A, Arya M, Shergill IS, Sharma D, Hammadeh MY, Mushtaq I. Current status of robotic surgery in pediatric urology. Pediatr Surg Int. 2008; 24:973–977. PMID: 18668251.

46. Atug F, Woods M, Burgess SV, Castle EP, Thomas R. Robotic assisted laparoscopic pyeloplasty in children. J Urol. 2005; 174:1440–1442. PMID: 16145459.

47. Kutikov A, Nguyen M, Guzzo T, Canter D, Casale P. Robot assisted pyeloplasty in the infant-lessons learned. J Urol. 2006; 176:2237–2239. PMID: 17070302.

48. Lee RS, Retik AB, Borer JG, Peters CA. Pediatric robot assisted laparoscopic dismembered pyeloplasty: comparison with a cohort of open surgery. J Urol. 2006; 175:683–687. PMID: 16407025.

49. Olsen LH, Rawashdeh YF, Jorgensen TM. Pediatric robot assisted retroperitoneoscopic pyeloplasty: a 5-year experience. J Urol. 2007; 178:2137–2141. PMID: 17870122.

50. O'Reilly PH, Brooman PJ, Mak S, Jones M, Pickup C, Atkinson C, et al. The long-term results of Anderson-Hynes pyeloplasty. BJU Int. 2001; 87:287–289. PMID: 11251517.
51. Ehrlich RM, Gershman A, Fuchs G. Laparoscopic vesicoureteroplasty in children: initial case reports. Urology. 1994; 43:255–261. PMID: 8116127.

52. Janetschek G, Radmayr C, Bartsch G. Laparoscopic ureteral anti-reflux plasty reimplantation. First clinical experience. Ann Urol (Paris). 1995; 29:101–105. PMID: 7645993.
53. Lakshmanan Y, Fung LC. Laparoscopic extravesicular ureteral reimplantation for vesicoureteral reflux: recent technical advances. J Endourol. 2000; 14:589–593. PMID: 11030542.
54. Gatti JM, Cartwright PC, Hamilton BD, Snow BW. Percutaneous endoscopic trigonoplasty in children: long-term outcomes and modifications in technique. J Endourol. 1999; 13:581–584. PMID: 10597129.

55. Cartwright PC. Percutaneous endoscopic trigonoplasty: a minimally invasive approach to correct vesicoureteral reflux. J Urol. 1996; 156(2 Suppl 1):661–664. PMID: 8683754.

56. Yeung CK, Sihoe JD, Borzi PA. Endoscopic cross-trigonal ureteral reimplantation under carbon dioxide bladder insufflation: a novel technique. J Endourol. 2005; 19:295–299. PMID: 15865516.

57. Kutikov A, Guzzo TJ, Canter DJ, Casale P. Initial experience with laparoscopic transvesical ureteral reimplantation at the Children's Hospital of Philadelphia. J Urol. 2006; 176:2222–2225. PMID: 17070297.

58. Lendvay T. Robotic-assisted laparoscopic management of vesicoureteral reflux. Adv Urol. 2008; 732942. PMID: 18682821.

59. Casale P, Patel RP, Kolon TF. Nerve sparing robotic extravesical ureteral reimplantation. J Urol. 2008; 179:1987–1989. PMID: 18355846.

60. Peters CA, Woo R. Intravesical robotically assisted bilateral ureteral reimplantation. J Endourol. 2005; 19:618–621. PMID: 16053348.

61. Casale P, Feng WC, Grady RW, Joyner BD, Lee RS, Mitchell ME. Intracorporeal laparoscopic appendicovesicostomy: a case report of a novel approach. J Urol. 2004; 171:1899. PMID: 15076303.

62. Pedraza R, Weiser A, Franco I. Laparoscopic appendicovesicostomy (Mitrofanoff procedure) in a child using the da Vinci robotic system. J Urol. 2004; 171:1652–1653. PMID: 15017258.

63. Thakre AA, Yeung CK, Peters C. Robot-assisted Mitrofanoff and Malone antegrade continence enema reconstruction using divided appendix. J Endourol. 2008; 22:2393–2396. PMID: 18937604.

64. Casale P, Grady RW, Feng WC, Joyner BD, Mitchell ME. A novel approach to the laparoscopic antegrade continence enema procedure: intracorporeal and extracorporeal techniques. J Urol. 2004; 171:817–819. PMID: 14713832.

65. Lendvay TS, Shnorhavorian M, Grady RW. Robotic-assisted laparoscopic mitrofanoff appendicovesicostomy and antegrade continent enema colon tube creation in a pediatric spina bifida patient. J Laparoendosc Adv Surg Tech A. 2008; 18:310–312. PMID: 18373465.

66. Docimo SG, Moore RG, Adams J, Kavoussi LR. Laparoscopic bladder augmentation using stomach. Urology. 1995; 46:565–569. PMID: 7571231.

67. Chung SY, Meldrum K, Docimo SG. Laparoscopic assisted reconstructive surgery: a 7-year experience. J Urol. 2004; 171:372–375. PMID: 14665934.

68. Lorenzo AJ, Cerveira J, Farhat WA. Pediatric laparoscopic ileal cystoplasty: complete intracorporeal surgical technique. Urology. 2007; 69:977–981. PMID: 17482947.

69. Gundeti MS, Eng MK, Reynolds WS, Zagaja GP. Pediatric robotic-assisted laparoscopic augmentation ileocystoplasty and mitrofanoff appendicovesicostomy: complete intracorporeal--initial case report. Urology. 2008; 72:1144–1147. PMID: 18804263.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download