Abstract
Purpose
We evaluated the efficacy of parenchymal compression in open partial nephrectomies (OPNs) compared with that of the conventional vascular clamping method.
Materials and Methods
OPNs were conducted by means of the parenchymal compression technique at our institution from April 2006. Among these, the operative outcomes of 20 consecutive patients with normal preoperative renal function (Group 1) were matched with those of 20 control patients from the database of previous operations who underwent OPN with a conventional vascular clamping method (Group 2).
Results
All preoperative characteristics were similar in both groups. The operative time was significantly higher for Group 2 (132.4±17.7 vs. 151.4±21.4 minutes, p=0.031). Estimated blood loss was slightly higher for Group 2, with marginal statistical significance (173.7±11.5 vs. 211.2±43.8 ml, p=0.06). Histologic examination revealed that over 80% of the tumors in both groups were renal cell carcinomas. For all patients, the pathology results of specimens were negative. Serum creatinine, checked at 1, 3, and 7 days after the operation, was significantly increased in both groups to a similar degree. However, 30 days after surgery, the patterns of serial serum creatinine levels demonstrated statistically significant differences by repeated-measures ANOVA (p<0.001), with a trend of more elevated in Group 2 than in Group 1, although values were within the normal range. No major complications occurred in either group.
Open nephron-sparing surgery is an accepted treatment modality for small renal masses less than 4 cm in size that provides oncological control comparable to that achieved with radical nephrectomy [1]. The goal of a partial nephrectomy is complete local excision of the focal lesion with minimal complications and optimal functional preservation of the renal remnant. Traditionally, partial nephrectomy is performed with renal pedicle clamping to facilitate tumor excision and decrease blood loss. However, renal vascular occlusion necessitates the dissection of the renal pedicle, which is technically challenging, time consuming, and potentially dangerous [2]. Furthermore, both renal vascular occlusion and the subsequent warm renal ischemia time increase the risk of postoperative renal failure [3].
Therefore, to facilitate the resection procedure and avoid hilar occlusion, a number of investigators have evaluated regional hypoperfusion using a variety of tourniquet-type methods or clamps [4-6]. Others have reported using a hand-assisted approach to allow for manual compression of the parenchyma [7,8]. These methods allow for the removal of renal tumors without clamping the renal pedicle, thus avoiding ischemia in the remaining renal tissue. However, despite favorable results with parenchymal compression methods, which have been reported as having shorter intra-operative times and less surgical blood loss, there have been no comparative studies with conventional vascular clamping methods directly addressing perioperative outcomes and maintenance of postoperative renal function. Here, we evaluated the efficacy of the parenchymal compression method by directly comparing perioperative variables, including operative time, blood loss, and serial changes to serum creatinine levels, with those of the conventional vascular clamping method.
Open partial nephrectomy (OPN) for small renal tumors less than 4 cm in size was performed by using one of two methods in our institution, according to the surgeon's preference. The first method used was the modified parenchymal compression method using umbilical tape (1/8" wide, Ethicone, USA). The second method was the traditional vascular clamping method. The parenchymal compression technique was used from April 2006; the indications for using this OPN technique were the presence of a small, exophytic, peripheral renal mass, including complicated cysts, located in a peripheral area of the kidney as evaluated by computed tomography (CT) or magnetic resonance imaging (MRI). We defined exophytic tumors as those where the lesion extended into more than 60% of the natural surface of the kidney [9]. A peripheral tumor location was defined as a tumor peripherally located in the kidney and enveloped by the renal cortical or medullary parenchyma with no extension into the renal sinus [10]. Due to relative surgical inaccessibility, this method was not used for hilar tumors, which were defined as tumors within 10 mm of the main vascular structure of the renal pedicle. Perioperative variables, including the American Society of Anesthesiology (ASA) score, body mass index (BMI), tumor size, tumor location, changes in serum creatinine, changes in hemoglobin, operative time, estimated blood loss (EBL), transfusion rates, length of hospital stay, pathology findings, and complication rates, were evaluated prospectively. Radiologic findings and serum creatinine, which was used as a surrogate for renal function, were also collected during the follow-up period. Among the patients undergoing this technique, 20 patients with a normal preoperative serum creatinine level, defined as below 1.5 mg/dl (Group 1), were exclusively enrolled in this study in an attempt to evaluate the effect of OPN in patients with normal preoperative renal function.
All patients who had OPN at our institution were not only registered prospectively in a specific database that includes all pertinent information about the tumor and patient demographic characteristics, but were also followed-up under the same strict guidelines. This allowed us to match the parameters of Group 1 patients against those of the other patient group, who had ORN with a conventional vascular clamping method. According to preoperative characteristics of the tumors and patients from a database collected from April 2004, 20 patients who had surgery with a vascular clamping method (Group 2) were carefully selected and enrolled in this series.
All procedures were performed by a single surgeon (DK Yoon). Regardless of the method used, the kidney was approached extraperitoneally from a standard flank incision between the 11th and 12th ribs in all cases. The kidney was mobilized while the perinephric fat overlying the tumor was left intact. In the modified parenchymal compression method using umbilical tape, the renal pedicle was not dissected. The renal parenchyma was surrounded selectively with the umbilical tape and was then compressed with a Kelly Mosquito clamp (Fig. 1). The umbilical tape was generally placed 2 to 3 cm from the tumor to allow for an excision with adequate surgical margins. To safeguard against inadvertent slippage of the umbilical tape, the line of renal parenchymal resection was 2 cm distal to the edge of the umbilical tape. The clamping pressure was carefully controlled manually by a second surgeon with a Mosquito clamp; the goal pressure was adequate to stop bleeding but not enough to damage the surrounding tissue. The transected parenchyma and surgical specimens were analyzed by cryosectioning in all cases. After identification of a negative surgical margin, the resected surface was then closed by using separated absorbable 2/0 sutures. In cases with positive surgical margins, more tissue was obtained from the previously resected site until identification of a negative surgical margin by cryosection. Any breach of the pelvocaliceal system was suture-repaired in a dry operative field. After adequate hemostasis, the umbilical tape was slightly relaxed. Further hemostasis was obtained, if necessary, and the umbilical tape was removed. After surgery, a closed drain was placed for all patients and left in place for 36 to 72 hours.
In cases using the conventional vascular clamping method, the renal artery was occluded with a bulldog vascular clamp; the renal vein was unclamped. Surface cooling was not conducted in any patients in this group. The resection margin was chosen as 1 cm distal to the tumor.
Patients were evaluated initially at one month postoperatively and then every 3 months during the first year, every 6 months during the second year, and then annually, with a medical history, physical examination, blood pressure, contrast-enhanced CT or MRI, chest X-ray, serum electrolytes, liver function tests, and serum creatinine tests. The radiologic finding of local recurrence was defined as a mass with strong enhancement at the excision site or in the perinephric space, with an increase in size on subsequent follow-up imaging [11].
All analyses were performed by using the SPSS program version 13.0. A p-value of less than 0.05 was considered statistically significant. For comparing each group, a Mann-Whitney U test and a chi-square test were used. For comparing serial changes in serum creatinine in each group, a repeated-measures ANOVA test, as an extension of the paired t-test to compare more than two repeated measures over time, was used.
The preoperative characteristics of the patients are summarized in Table 1. The two groups were similar in all preoperative variables. The mean±SD age and tumor size was 55.6±10.5 years old and 2.2±0.9 cm in Group 1 and 49.3±14.2 years and 2.31±1.0 cm in Group 2. The preoperative serum creatinine was 0.87±0.29 mg/dl in Group 1 and 0.91±0.24 mg/dl in Group 2.
The postoperative outcomes are summarized in Table 2. The operative time was significantly less in Group 1 than in Group 2 (132.4±17.7 vs. 151.4±21.4 minutes, p=0.03). EBL was slightly higher in Group 2, with borderline statistical significance (173.7±11.5 vs. 211.2±43.8 ml, p=0.06); consequently, the postoperative decrease in hemoglobin was lower in Group 1 (1.29±1.18 vs. 2.11±1.08 g/dl, p=0.06). One patient in Group 1 and three patients in Group 2 needed postoperative transfusions. Histologic examination revealed that over 80% of the tumors in both groups were renal cell carcinomas. For all patients, the pathology results of the initial frozen specimens were negative; the final pathologic report reconfirmed the initial results obtained by cryosectioning.
The serum creatinine level during the surgical admission increased at postoperative day one and then gradually decreased. Serum creatinine levels, checked at 1, 3, and 7 days after the operation, were significantly increased in both groups to a similar extent. However, 30 days after surgery, the pattern of serial serum creatinine levels in each group demonstrated statistical differences in repeated-measures ANOVA testing (p<0.001). In Mann-Whitney U testing, the serum creatinine levels measured 30 days after surgery were statistically similar to preoperative levels in Group 1 (p=0.56), although they were still elevated in Group 2 (p<0.001). Similar differences between preoperative and postoperative serum creatinine levels were found at 90, 180, and 360 days postoperatively (Fig. 2).
Inadvertent slippage of the U-umbilical tape loop did not occur in any patients in Group 1. No major complications, including conversion to nephrectomy or major vascular injury, occurred in either group. Three patients had minor complications: one patient in each group experienced urine leakage, but this was resolved after insertion of a temporary ureteral stent; one patient in Group 2 experienced a perirenal hematoma, which completely subsided, as determined by CT imaging, 3 months postoperatively. During a mean follow-up of 24.1±10.6 (13-47) months and 26.66±14.9 (16-48) months for the renal cell carcinoma patients in Groups 1 and 2, respectively, there were no cases of metastasis or disease recurrence. As determined by radiologic imaging, no patients experienced hydronephrosis. No patients required hemodialysis at any time after surgery.
The number of patients undergoing partial nephrectomy has increased due to the changing indications for this type of surgery, as well as the increased detection of incidental, small renal tumors [12]. A partial nephrectomy is a technically demanding procedure that may lead to serious complications if not performed correctly. Hemostasis of the resected surface is one of the most important aspects of partial nephrectomy surgery. Intraoperative and delayed bleeding with the necessity of a secondary nephrectomy have been reported as complications of this procedure [13]. Therefore, a partial nephrectomy usually involves a wide dissection of the renal pedicle and artery, vein clamping, and renal cooling, all to provide better bleeding control. However, pedicle dissection is time-consuming and associated with a risk of vascular injury and profuse bleeding. Renal pedicle clamping limits the available resection time to 30-60 min, during which time the lesion must be removed, prominent vessels sutured, the collecting system closed, and tissue removed for cryosection analysis. The most serious complications of this conventional surgical method are urinary fistulae (8-10%) and acute renal failure, especially in 20-25% of patients having a solitary kidney [14]. In these cases, hemodialysis is necessary until kidney function has recovered sufficiently. Ischemic renal failure following partial nephrectomy has been attributed to prolonged clamping of the renal artery leading to ischemia of the entire renal mass, intraoperative hypotension, and, rarely, postoperative thrombosis of the renal artery or vein [14,15].
In light of these potential limitations, many alternative surgical methods have been developed in an attempt to decrease complication rates and improve renal function. After the initial introduction by Gill et al [6], many other methods have been introduced, including the use of the Reni-Clamp [2], the Satinsky clamp [16], vascular clamps [17], and the large curved DeBakey aortic clamp [18,19]. Gill et al, who used the tourniquet method, similar to the method we use here, reported that because the renal hilum was not occluded, blood flow to the remainder of the kidney was uninterrupted, thus minimizing the chances of ischemic injury to the renal remnant. They also reported that the use of the double loop apparatus eliminated the need for surgical mobilization of the renal hilar vessels. Furthermore, because renal hypothermia was not required, the kidney did not need to be packed in ice slush intraoperatively. Tsivian and Sidi used a 1 cm wide Vicryl mesh strip for parenchymal compression and reported that the resultant blood loss was minimal; the maximal blood loss reported was 200 ml [20]. Among 61 patients undergoing a partial nephrectomy with this method, only one patient developed gross hematuria; this resolved after conservative treatment. Huyghe et al [2], who used the Reni-Clamp, reported the results of open partial nephrectomy in 33 patients. The mean operative and clamping times were 150 and 27 minutes, respectively; the EBL was 150 (range, 50-450) ml. Postoperatively, no blood transfusions were required. Similarly, Mejean et al [18], who used a DeBakey aortic clamp in 10 patients with renal cell carcinoma, reported insignificant intraoperative blood loss with no complications, including urinary flank drainage or renal dysfunction. In the present series of patients using umbilical tape parenchymal compression, benefits consistent with those previously reported were noted when compared with the standard vascular clamping method. The total operative time was significantly lower in the parenchymal compression group. Although not statistically significant, EBL was lower in the parenchymal compression group with a concurrent lower decrease in hemoglobin levels after surgery. Because there was no need for dissection around the hilar vasculature using the parenchymal compression technique, the operative time for this approach was reduced. Due to the anatomic complexity around the hilar vessels, the potential risk for bleeding was also decreased with the parenchymal compression technique. Additionally, there was no risk of venous bleeding with the use of parenchymal compression because there was occlusion of the renal artery only. Thus, we propose that the relative technical simplicity of the parenchymal compression technique positively affects surgical outcomes.
One of the potential advantages of using the parenchymal clamping method compared with conventional vascular clamping is its positive effect on preservation of renal function. This method enables one to avoid warm ischemic time in the affected kidney. Rodríguez-Covarrubias et al [19], who used the DeBakey aortic clamp in seven patients with normal preoperative renal function, including one solitary kidney case, reported that the mean preoperative serum creatinine level was 0.74 mg/dl (range, 0.58-1.26 mg/dl) and the mean postoperative serum creatinine level was 0.81 mg/dl (range, 0.69-1.21 mg/dl); no patients required dialysis. In the current study, with a minimum follow-up of 12 months, although serum creatinine was temporarily increased during surgical admission in both groups, the pattern of serial changes in serum creatinine was significantly different between the groups. There was a trend toward an elevated serum creatinine in Group 2 compared with Group 1 (Fig. 2). However, different from previous reports of an unchanged serum creatinine by use of the conventional vascular clamping method, the increased serum creatinine level observed in Group 2 in this study merits discussion. Although slippage of the umbilical tape did not occur in the current series, it is a critically important possibility to bear in mind when using the parenchymal compression technique. Thus, although we attempted to position the umbilical tape in such a way as to be able to obtain a 2-3 cm margin from the tumor itself, the actual resection point using this technique tended to be minimized when compared with that of the vascular clamping method (where we attempted to obtain a resectional margin approximately 1 cm from the tumor). Although the size of the surgical margin in OPN does not seem currently to affect the risk of local tumor recurrence [21], the patients enrolled in Group 2 were a historical cohort. Thus, this group contained patients undergoing OPN with a different surgical margin policy, namely, the further the resection from the tumor margin, the greater advantage there was in tumor control. Due to this policy, the amount of normally functional renal tissue resected in Group 2 would be expected to be larger than in Group 1; this may have affected the postoperative serum creatinine levels.
Despite positive findings in terms of perioperative and renal function outcomes, we recognize some limitations of this study series. This technique cannot be applied to all renal tumors, as indicated by Joung et al [22]. To obtain sufficient compression of the kidney, it is imperative to circumscribe the mass with umbilical tape. However, for some tumors located in the hilar space, being endophytic and central, rather than exophytic and peripheral, makes it difficult to perform this technique. Whereas our data included five cases of midpole lesions, all of these were small, peripherally located lesions; this allowed for complete settlement of the umbilical tape in an oblique fashion. Other limitations were intrinsic to the study design. Although we attempted to collect data prospectively, the entire study design was not defined at the time of initiation. The margin goal was not initially standardized in our study, as previously mentioned, which may have negatively affected the serum creatinine levels in Group 2. Also, serial serum creatinine measurements were used as a surrogate of renal function for simplicity, although this measure is not an accurate indicator for practical renal function. Whereas differences in the pattern of serial serum creatinine measurements were noted, the increased serum creatinine levels were still within normal limits. This observation leaves unanswered the role of renal function preservation by use of the parenchymal compression technique in patients with abnormal renal function. Whereas these data demonstrated practical advantages in terms of operative time, without compromising oncologic outcomes or increased complication rates, in order to evaluate the role of this technique on actual renal function, a study measuring serial glomerular filtration rates and having a strict operative procedural design is needed.
OPN using the parenchymal compression method had acceptable outcomes in terms of complete tumor control, avoidance of warm ischemic time, and minimizing blood loss, with good preservation of renal function and minimal complications. Therefore, in selected tumors with peripheral nonhilar localization, this can be an alternative approach to traditional vascular clamping approaches.
Figures and Tables
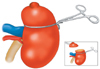 | FIG. 1The modified renal parenchymal compression method using umbilical tape. The renal parenchyma was surrounded selectively with the umbilical tape, which was generally placed 2-3 cm from the tumor to allow for an excision with adequate surgical margins and was then compressed with a Mosquito clamp. To safeguard against inadvertent slippage of the umbilical tape, the line of renal parenchymal resection was 2 cm distal to the edge of the umbilical tape. |
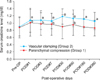 | FIG. 2Serial changes in serum creatinine in the two groups during the surgical hospitalization and follow-up periods. a: significant increase in the postoperative serum creatinine level compared with the preoperative level in the vascular clamping group (Group 2), by Mann-Whitney U test, b: significant increase in the postoperative serum creatinine level compared with the preoperative level in the parenchymal compression group (Group 1), by Mann-Whitney U test. |
References
1. Novick AC. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Open surgery of the kidney. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;1720–1732.
2. Huyghe E, Nohra J, Leobon B, El Khoury E, Khedis M, Soulié M, et al. Open partial nephrectomy with selective renal parenchymal control: a new reliable clamp. Urology. 2006. 68:658–660.
3. Campbell SC, Novick AC, Streem SB, Klein E, Licht M. Complications of nephron sparing surgery for renal tumors. J Urol. 1994. 151:1177–1180.
4. McDougall EM, Clayman RV, Chandhoke PS, Kerbl K, Stone AM, Wick MR, et al. Laparoscopic partial nephrectomy in the pig model. J Urol. 1993. 149:1633–1636.
5. Cadeddu JA, Corwin TS, Traxer O, Collick C, Saboorian HH, Pearle MS. Hemostatic laparoscopic partial nephrectomy: cable-tie compression. Urology. 2001. 57:562–566.
6. Gill IS, Munch LC, Clayman RV, McRoberts JW, Nickless B, Roemer FD. A new renal tourniquet for open and laparoscopic partial nephrectomy. J Urol. 1995. 154:1113–1116.
7. Stifelman MD, Sosa RE, Nakada SY, Shichman SJ. Hand-assisted laparoscopic partial nephrectomy. J Endourol. 2001. 15:161–164.
8. Brown JA, Hubosky SG, Gomella LG, Strup SE. Hand assisted laparoscopic partial nephrectomy for peripheral and central lesions: a review of 30 consecutive cases. J Urol. 2004. 171:1443–1446.
9. Venkatesh R, Weld K, Ames CD, Figenshau SR, Sundaram CP, Andriole GL, et al. Laparoscopic partial nephrectomy for renal masses: effect of tumor location. Urology. 2006. 67:1169–1174.
10. Hafez KS, Novick AC, Butler BP. Management of small solitary unilateral renal cell carcinomas: impact of central versus peripheral tumor location. J Urol. 1998. 159:1156–1160.
11. Lee MS, Oh YT, Han WK, Rha KH, Choi YD, Hong SJ, et al. CT findings after nephron-sparing surgery of renal tumors. AJR Am J Roentgenol. 2007. 189:W264–W271.
12. Smith SJ, Bosniak MA, Megibow AJ, Hulnick DH, Horii SC, Raghavendra BN. Renal cell carcinoma: earlier discovery and increased detection. Radiology. 1989. 170:699–703.
13. Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, methods and outcomes. J Urol. 2001. 166:6–18.
14. Ghavamian R, Cheville JC, Lohse CM, Weaver AL, Zincke H, Blute ML. Renal cell carcinoma in the solitary kidney: an analysis of complications and outcome after nephron sparing surgery. J Urol. 2002. 168:454–459.
15. Licht MR, Novick AC. Nephron sparing surgery for renal cell carcinoma. J Urol. 1993. 149:1–7.
16. Denardi F, Borges GM, Silva W Jr, Stopiglia RM, Ferreira U, Billis A, et al. Nephron-sparing surgery for renal tumours using selective renal parenchymal clamping. BJU Int. 2005. 96:1036–1039.
17. Cariou G, Cussenot O. Hemostasis technics in partial nephrectomy. Prog Urol. 1996. 6:605–606.
18. Mejean A, Vogt B, Cazin S, Balian C, Poisson JF, Dufour B. Nephron sparing surgery for renal cell carcinoma using selective renal parenchymal clamping. J Urol. 2002. 167:234–235.
19. Rodríguez-Covarrubias F, Gabilondo B, Borgen JL, Gabilondo F. Partial nephrectomy for renal tumors using selective parenchymal clamping. Int Urol Nephrol. 2007. 39:43–46.
20. Tsivian A, Sidi AA. A simple and reliable hemostatic technique during partial nephrectomy. Urology. 2004. 63:976–978.
21. Lam JS, Bergman J, Breda A, Schulam PG. Importance of surgical margins in the management of renal cell carcinoma. Nat Clin Pract Urol. 2008. 5:308–317.
22. Joung JY, Jeong IG, Han KS, Yang SO, Jo YJ, Lee KH, et al. Partial nephrectomy using parenchymal compression without renal pedicle clamping. Korean J Urol. 2007. 48:265–269.
Advances in operative techniques and instruments have made nephron-sparing surgery for the renal cell carcinoma a surgical alternative for small tumors of less than 4 cm in the setting of a normal contralateral kidney. Oncologic outcomes have been reported to be no different than those in patients undergoing radical nephrectomy, a finding corroborated across multiple institutions [1-3]. However, possible operative complications, including hemorrhage, urine leakage, and renal ischemia, are a concern. Temporary hilar clamping, a method to prevent operative bleeding, results in warm ischemia. An association has been noted be tween hilar clamping time and changes in renal function [4]. Some authors perform a parenchymal compression in open partial nephrectomy to spare renal function.
This article evaluated the efficacy of a parenchymal compression method using umbilical tape in open partial nephrectomies. It showed a good preservation of postoperative renal function with minimal operative complications. I support the conclusion that the compression method might have a role in preventing bleeding and saving renal function. Nevertheless, I would like to make a few comments on this article. First, the method used to evaluate ischemic renal function was not complete. The level of serum creatinine is meaningless as long as there is a normal contralateral kidney. Nevertheless, this article relied on serum creatinine levels only. The authors should consider investigating split renal function, such as by MAG3 renal scan or the glomerular filtration rate [5]. Second, some authors have reported a compression method using a Kauffman clamp and DeBakey aortic clamp, which is presented in this article. The authors should explain the advantages of the umbilical tape compared with previously reported methods and its usage for midpole tumors. Third, the follow-up period of 24 (range, 13-47) months is not long enough for complete cancer control. Long-term follow-up results are needed to confirm the cancer control effect.
Despite my comments, this article indicates the feasibility and acceptable operative results of the compression method in open partial nephrectomy.
References
1. Lerner SE, Hawkins CA, Blute ML, Grabner A, Wollan PC, Eickholt JT, et al. Disease outcome in patients with low stage renal cell carcinoma treated with nephron sparing or radical surgery. J Urol. 1996. 155:1868–1873.
2. Patard JJ, Shvarts O, Lam JS, Pantuck AJ, Kim HL, Ficarra V, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004. 171:2181–2185.
3. Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000. 163:442–445.
4. Thompson RH, Leibovich BC, Lohse CM, Zincke H, Blute ML. Complications of contemporary open nephron sparing surgery: a single institution experience. J Urol. 2005. 174:855–858.
5. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006. 354:2473–2483.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download